Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is a clinicopathologic entity that requires a liver biopsy assessment to diagnose the progressive form of NAFLD called non-alcoholic steatohepatitis (NASH). Liver biopsy is invasive, subject to sampling and interobserver variability, and impractical to scale to the affected population of up to 1 billion affected individuals worldwide. Non-invasive imaging biomarkers have emerged as a key modality to address the major unmet need to diagnose, stage, and longitudinally monitor NAFLD.
Scope of review
In this review, we critically examine the use of non-invasive imaging biomarkers to diagnose NAFLD, NASH, and fibrosis stage.
Major Conclusions
Ultrasound and magnetic resonance imaging (MRI) biomarkers of liver fat can diagnose NAFLD. MRI proton density fat fraction (MRI-PDFF) is better than liver biopsy, particularly for following longitudinal changes in liver fat in clinical trials. Imaging biomarkers to reliably diagnose NASH are under investigation, but when used alone, continue to have only modest diagnostic accuracy. However, the fibrosis stage has the strongest association with liver decompensation and mortality, and elastography has emerged as a reliable biomarker for liver fibrosis. We review the combination of biomarkers to risk stratify patients and identify individuals needing treatment and the implications of longitudinal changes in liver stiffness measurement.
Keywords: NAFLD, MRI, NASH, Significant fibrosis, MR Elastography, MRI-PDFF
Highlights
-
•
An improvement of ≥30% in liver fat on MRI-PDFF is associated with histologic improvement.
-
•
Combining MRE ≥3.3 kPa and FIB-4 ≥ 1.6 (MEFIB Index) predicts high-risk NAFLD.
-
•
Elevated liver stiffness measurements predict future hepatic decompensation.
-
•
MRE ≥ 4.67 kPa and ≥8 kPa predict cirrhosis and hepatic decompensation, respectively.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined by the presence of excessive accumulation of liver fat in the absence of liver fat accumulation secondary to other causes, including excessive alcohol use, steatogenic medications, or other causes of concomitant liver disease [1]. NAFLD is subdivided into two primary subtypes, non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH), which is considered the progressive form of NAFLD. Distinguishing between NAFL and NASH depends on identifying pathologic features of liver histology, namely steatosis plus lobular inflammation and hepatocyte ballooning typically in zone 3 with or without peri-sinusoidal fibrosis [2]. However, the development of liver fibrosis is most strongly associated with morbidity and mortality [3]. Significant fibrosis, defined as stage 2 or greater, is associated with an increased risk of liver-related and all-cause mortality [3]. Therefore, individuals with the greatest need of therapeutic interventions are those with NASH with significant fibrosis.
NAFLD is increasingly common with rising prevalence in parallel with the global epidemic of obesity-related metabolic syndrome [4,5]. NAFLD is closely associated with metabolic risk factors, and a panel of experts recently advocated changing the definition to metabolic dysfunction-associated liver disease (MAFLD) to require the presence of metabolic dysregulation and include overlap with other liver diseases; however, the new definition has not been broadly adopted [6]. NAFLD is a heterogeneous disease, and while cardiovascular disease is the leading cause of mortality, certain genetic polymorphisms that predispose to liver disease may protect against cardiovascular disease [7]. In the US, NAFLD is the second leading indication for liver transplantation [8] and an increasingly common cause of hepatocellular carcinoma [[9], [10], [11]]. While the global prevalence of NAFLD is projected to be 25%, only a subset of the affected population is at risk for progression. Liver biopsy is the current reference standard and required to distinguish NAFL from NASH but is impractical to scale to the affected population and invasive, costly, and results in high intra- and interobserver variability even among expert pathologists [12,13].
It is in this context that a significant need for non-invasive biomarkers of NAFLD has emerged. Biomarkers must address three major domains; (1) quantifying liver fat to diagnose NAFLD, (2) assessing disease severity, namely the degree of inflammation and fibrosis, and (3) successfully exploring longitudinal changes over time. The context of biomarker development in NAFLD has been limited by liver biopsy serving as an imperfect reference standard. In addition, there is often a long latency period from diagnosis to objective outcomes of liver-related morbidity and mortality. Despite these challenges, imaging biomarkers have emerged as reliable surrogates for diagnosing and assessing disease severity. Emerging data also support their use to explore longitudinal changes in disease activity over time. In this review, we evaluate the role of non-invasive imaging biomarkers to diagnose NAFLD by quantifying liver fat and assessing disease severity including identifying the populations in greatest need of treatment. We also review emerging data on using non-invasive imaging biomarkers to assess changes in NAFLD activity and severity and the complimentary combination of blood-based tests with imaging to increase diagnostic accuracy.
2. Imaging biomarkers of liver fat
The identification of pathologic liver fat, ≥5%, is required to diagnose NAFLD. Currently, the diagnosis of fatty liver is often incidental or found on examination of elevated liver enzymes. Conventional ultrasound and computed tomography (CT) have poor sensitivity for detecting mild levels of steatosis in NAFLD [14,15], and given that liver fat decreases with advanced fibrosis/cirrhosis [16], these modalities may miss the population in greatest need of identification and intervention. CT also exposes patients to ionizing radiation, thereby increasing the risk associated with its use as a repeated measure of liver fat.
2.1. Ultrasound-based biomarkers of liver fat
While conventional ultrasonography utilizes semi-quantitative ordinal categories of liver fat as mild, moderate, and severe with poor interobserver agreement [17], newer quantitative ultrasound-based techniques demonstrate superior performance. These methods leverage quantitative data based on the acoustic parameters of liver tissue. The attenuation parameter, backscatter coefficient, and speed/wavelength of ultrasonic wave data are the most well-evaluated quantitative characteristics [18]. Furthermore, combining these parameters may lead to increased diagnostic accuracy [19].
The controlled attenuation parameter (CAP) is being increasingly utilized and implemented as an ultrasound-based tool to assess liver fat and can be obtained simultaneous with a liver stiffness measurement (LSM) by vibration-controlled transient elastography (VCTE) commercially marketed as FibroScan. Measurements are recommended to be obtained on patients in the supine position after at least a 2-h fast. Two probes, M and XL, are available and automatic probe selection software included in the device recommends using the XL probe when the skin to liver capsule distance is > 25 mm. Advantages of CAP include that it is a rapid, point-of-care assessment with good sensitivity and specificity for diagnosing fatty liver [20]. In addition, CAP is obtained simultaneously with VCTE. Despite these strengths, specific limitations of CAP include identifying the optimal cut point, implementing criteria for validity, and the impact of probe selection.
Karlas et al. conducted an individual patient meta-analysis on CAP diagnostic accuracy with liver biopsy as the reference and described 248 dB/m as the optimal cut point for identifying fatty liver [20]. Importantly, the majority of patients in the study had non-NAFLD liver disease, and subsequent studies demonstrated higher optimal cut points in well-characterized NAFLD populations. Specifically, Caussy et al. evaluated the optimal CAP cut point for fatty liver with magnetic resonance imaging proton density fat fraction (MRI-PDFF) as the reference and identified 288 dB/M [21], which was then replicated in a population of HIV-associated NAFLD [22]. Eddowes et al. defined the optimal cut point as 302 dB/M with liver histology as the reference [23]. Importantly, while CAP could distinguish mild from moderate to severe steatosis, it has limited reliability to discriminate between moderate and severe steatosis. Furthermore, CAP measurements with a high interquartile range (IQR) have demonstrated reduced validity in studies with histology [24] or MRI-PDFF [21] as the reference standard. Probe selection also impacts CAP values, and optimal cut points for the diagnosis of fatty liver are lower using the M probe vs the XL probe [25]. Taken together, interpreting CAP measurements should consider the probe choice and validity and are a reasonable point-of-care diagnostic for the presence or absence of fatty liver disease.
2.2. Magnetic resonance-based biomarkers of liver fat
Magnetic resonance spectroscopy (MRS) measures proton signals as a function of their resonance frequency to separate fat and water signal fractions. MRS can detect small amounts of liver fat and is considered the most accurate non-invasive method to quantify liver fat [26,27]. However, MRS has significant limitations that prevent its widespread use including the need for specialized expertise to perform and analyze MRS as well as possible sampling errors with restricted spatial coverage. MRI-based assessment of liver fat also utilizes the difference in resonance frequencies between fat and water. MRI-PDFF corrects for imaging confounders that can affect liver fat measurement and has emerged as an accurate, reproducible biomarker of liver fat [[28], [29], [30], [31], [32], [33], [34], [35]]. Importantly, MRI-PDFF overcomes the limitations of MRS by being widely available in commercial MRI systems with PDFF maps automatically reconstructed. Furthermore, MRI-PDFF maps allow for assessing multiple regions of interest (ROI) and following them longitudinally, which can overcome the heterogeneity of liver fat deposition. MRI-PDFF is reported as a continuous measure from a direct measurement of physical properties of the liver, whereas liver fat on a liver biopsy is a visual estimate reported in broad ordinal categories. In a longitudinal assessment, MRI-PDFF was more accurate at detecting changes in liver fat than liver biopsy [36] and has been validated in multiple studies [37].
Head-to-head comparisons have demonstrated that MRI-PDFF outperforms CAP in diagnostic accuracy with steatosis grade on liver biopsy as the reference [33,38]. Currently, the main utility of CAP is diagnosing fatty liver, whereas MRI-PDFF provides additional accurate quantification of the amount of liver fat that may be useful for evaluating a response to an intervention and is frequently deployed in clinical trials. Further studies on the prognostic significance of high liver fat on MRI-PDFF and its cost-effectiveness will be required prior to broadly implementing its use in clinical practice.
3. Association between MRI-PDFF and histologic response in NASH
These advantages have led to implementing MRI-PDFF as a key diagnostic and longitudinal biomarker in NAFLD. In addition to aiding the diagnosis of NAFLD, the amount of liver fat on MRI-PDFF may be associated with disease activity and progression. A proof-of-concept study demonstrated that higher liver fat on MRI-PDFF was associated with fibrosis progression [39] in patients with sequential liver biopsies but will require validation in larger cohorts. Longitudinal change in MRI-PDFF has been used as an endpoint in multiple phase II clinical trials [30,40,41], particularly when the drug is likely to have an anti-steatotic effect [42]. Furthermore, MRI-PDFF improvement by ≥ 30% has been associated with improved liver histology. Stine et al. conducted a meta-analysis of seven studies demonstrating that a ≥30% relative decline in MRI-PDFF is associated with 6.98 (95% CI 2.38–20.43, p < 0.001) times higher odds of ≥ 2-point improvement in NAFLD activity scores and 5.45 (95% CI: 1.53–19.46) times higher odds of histologic NASH resolution compared to non-responders [43].
4. Imaging biomarkers of NASH and fibrosis
4.1. Diagnosis of NASH
Current imaging-based biomarkers have limited diagnostic accuracy for NASH [44]. Two primary domains of imaging biomarkers have been evaluated to address this need, corrected T1 (cT1) MRI and elastography. T1 relaxation time on MRI is a function of extracellular fluid that is associated with inflammation and fibrosis. By correcting for the opposing effects of T2∗ sequences, a cT1 value can be derived, which is associated with histologic inflammation and fibrosis with c-statistic for NASH of 0.80, but limited specificity, 52%, in a small study of 71 patients with suspected NAFLD and liver biopsy [45]. Importantly, cT1 cannot discriminate fibrosis and inflammation, limiting the ability to distinguish NASH from fibrosis, but may be associated with long-term liver-related outcomes [46]. Conversely, elastography measures the elasticity of an object and in liver disease is closely associated with the degree of fibrosis. Evaluation of 2-dimensional (2D) magnetic resonance elastography (MRE) and 3-dimensional (3D) MRE revealed excellent diagnostic accuracy for fibrosis, as detailed to follow, but only modest ability to discriminate NAFL from NASH c-statistics 0.73 and 0.75, respectively [47,48]. Multiparametric MRE is a novel imaging approach that combines assessment at multiple frequencies with 3D MRE to provide a more comprehensive analysis of liver stiffness but also has limited diagnostic accuracy for NASH, c-statistic 0.73 [49]. Similar attempts to diagnose NASH using VCTE at a cutoff of 7 kPa have also yielded modest diagnostic accuracy, c-statistic 0.75 [50].
4.2. Ultrasound-based biomarkers of fibrosis
Ultrasound-based measurements of liver stiffness can be integrated into conventional ultrasound devices as in acoustic resonance-forced impulse imaging (ARFI) and shear wave elastography (SWE) or obtained through a dedicated device, most commonly VCTE, which is commercially available as FibroScan. ARFI and SWE use high-frequency ultrasound impulses to generate sheer waves and require the operator to define a region of interest and obtain a series of liver stiffness measurements. Limited studies of SWE and ARFI in patients with NAFLD have demonstrated very good diagnostic accuracy for advanced fibrosis [51,52]. However, additional data on the optimal cut points and quality criteria are needed.
VCTE provides a point-of-care liver stiffness measurement using a single hand-held probe that measures the velocity of low-amplitude shear waves using ultrasound. As previously detailed regarding CAP, an M probe and XL probe are both available and the XL probe measures wave propagation at greater depths to overcome the impact of larger chest wall depths often from adiposity in NAFLD patients. Implementing the XL probe decreased the failure rate associated with FibroScan in NAFLD patients [53]. VCTE has been evaluated extensively leading to the development of quality criteria to guide its use. Specifically, a minimum of 10 valid measurements of which >60% should be valid and the ratio of the median valid liver stiffness measurement to IQR should be less than or equal to 0.3 [54]. Ongoing limitations of VCTE in NAFLD include (1) unclear optimal cut points, (2) inability to scan or an unreliable scans particularly in the morbidly obese or with an inexperienced operator, (3) limited publications utilizing the XL probe, and (4) limited diagnostic accuracy for earlier stages of fibrosis. Importantly, studies reporting cut-offs with fixed sensitivity and fixed specificity illustrate the wide range of measured liver stiffness values for each histologic fibrosis stage. Specifically, a multicenter report from the NASH Clinical Research Network using the M and XL probes demonstrated a low failure rate and high c-statistic for advanced fibrosis [55]. However, to exclude advanced fibrosis at a fixed sensitivity (0.90), a cut-off of 6.5 kPa is used, and to have a high specificity (0.90), a cut-off of 12.1 kPa is required. The optimal cut point using Youden's index of 8.6 is similar to other reports [56] but yielded a low PPV, 0.59, in the study.
4.3. MRI-based biomarkers of fibrosis
Magnetic resonance elastography (MRE) utilizes a mechanical driver to generate shear waves that can be assessed through a modified pulse sequence. This requires the addition of hardware (driver) and proprietary software. Wave images are converted to elastograms, which are cross-sectional maps of liver stiffness. Then a region of interest free of vessels can be selected and a mean liver stiffness measurement in kPa reported. Importantly, despite using the same units, kPa, results of liver stiffness measurements are not interchangeable between different modalities. MRE has a low failure rate [57] and is less affected by obesity with excellent interobserver agreement [58,59]. However, acute inflammation and iron overload can be associated with overestimations of liver stiffness and failed examinations, respectively.
MRE is most often performed at 60 Hz in 2D with excellent diagnostic accuracy for advanced fibrosis at a cut point of 3.63 kPa (c-statistic > 0.90) [33,38,56,60]. However, 3D-MRE can evaluate a larger volume of the liver and may increase the diagnostic accuracy of MRE for advanced fibrosis [48]. Currently, 3D MRE requires significant expertise and further validation before implementation in clinical practice. Importantly, MRE outperforms VCTE in head-to-head comparisons and has a high diagnostic accuracy for earlier stages of fibrosis, including patients with significant fibrosis who are at risk of disease progression and are potential candidates for treatment [33,56]. However, MRE may not be tolerated in a subset of patients who may be too obese for the MRI scanner, have incompatible metallic implants, or have claustrophobia. MRI is more costly than VCTE; however, a cost-effectiveness analysis for detecting cirrhosis in patients with NAFLD demonstrated that FIB-4 + MRE was more cost-effective than proceeding to biopsy after FIB-4 [61]. Further studies of the cost-effectiveness of diagnostic strategies using MRE to diagnose earlier stages of fibrosis are needed.
5. Future directions and emerging data
As our understanding of the strengths and weaknesses of imaging biomarkers evolves, multiple areas remain that require further research to fill the unmet needs for diagnosing, staging, and monitoring NAFLD. Specifically, the optimal combination or sequential use of imaging and blood-based biomarkers needs to be defined, particularly in specific contexts of use. For example, the optimal combination of biomarkers used in a general or primary care practice to guide specialty referral may be different than those used to evaluate for biopsy in a specialty practice or consideration of screening for a clinical trial. Despite extensive study, we have limited data on optimal cut points and quality criteria in many modalities of non-invasive imaging biomarkers. There are only limited data on the association with long-term outcomes and implications of a change in a non-invasive imaging biomarker.
5.1. Non-invasive biomarkers to identify those in need of treatment
The combinatorial use of biomarkers can be used in a complementary manner to non-invasively identify patients with NAFLD with significant fibrosis and NASH who would benefit most from treatment. Newsome et al. combined VCTE, CAP, and aspartate aminotransferase (AST) in the FAST score and demonstrated good diagnostic accuracy for patients with significant fibrosis and a histologic NAFLD activity score of ≥4 [62]. The test was validated in multiple international cohorts and used an upper and lower cut point; however, 30–40% of the population falls into an indeterminate gray zone. In addition, combining the blood-based enhanced liver fibrosis (ELF) score ≥ 9.8 and VCTE ≥ 14.0 kPa was demonstrated to have a PPV of 87% for stages 3–4 fibrosis in a secondary analysis of the ATLAS trial.
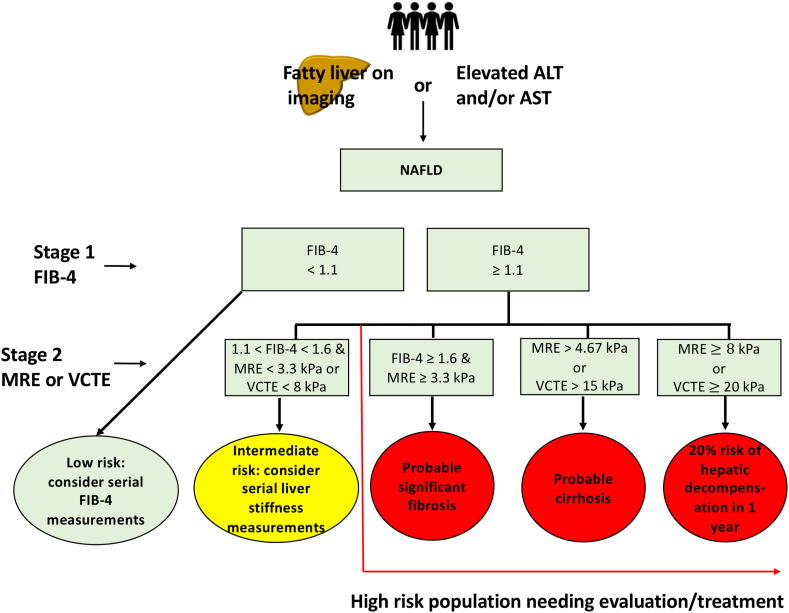
Detection of ≥ stage 2 fibrosis remains a major unmet need. One of the major limitations in the past has been a high NPV but low PPV to rule in patients who are candidates for treatment in NASH (≥2 stage fibrosis). A prospective study including 460 patients who underwent contemporaneous MRE, FIB-4, and liver biopsy assessment demonstrated that combining MRE ≥3.3 kPa and FIB-4 ≥ 1.6 (MEFIB Index) provided an excellent PPV of 97.1% for significant fibrosis and was validated in an independent and ethnically and geographically diverse cohort from Japan. The simultaneous combination of multiple biomarkers is well suited to enriching populations needing treatment, particularly for clinical trials. However, the sequential use of non-invasive tests will likely prove more feasible for broad implementation and minimize indeterminate results [63]. We propose an algorithm for sequential use of non-invasive tests for patients with suspected NAFLD (Figure 1).
Figure 1.
Sequential use of FIB-4 and VCTE/MRE for risk stratification in NAFLD.
5.2. Longitudinal assessment of liver stiffness
Higher baseline liver stiffness [64] and changes in liver stiffness were recently demonstrated to be associated with liver-related and all-cause mortality in multiple studies. VCTE data on 1,039 NAFLD patients with histologically advanced fibrosis or VCTE >10 kPa found that baseline and changes in liver stiffness values, available in 533 patients, were associated with hepatic decompensation, HCC, overall mortality, and liver-related mortality [65]. Importantly, the risk of decompensation increased in a dose-dependent fashion based on the change in liver stiffness over time, ranging from 3.8% in those with a >20% decrease in liver stiffness to 14.4% in those with a >20% increase in liver stiffness (Table 1). Similarly, Han et al. demonstrated that higher baseline liver stiffness on MRE was associated with hepatic decompensation [66]. Gidener et al. recently demonstrated baseline LSM on MRE also predicts future decompensation and death. They then used longitudinal outcome data on the development of cirrhosis to guide the optimal interval between non-invasive monitoring [67]. Regarding changes in MRE, a secondary analysis of 54 patients in a phase II trial of selonsertib demonstrated that any improvement in MRE was associated with improved fibrosis albeit with a limited diagnostic accuracy [68]. A separate longitudinal study with paired biopsy demonstrated that a 15% increase in MRE was clinically significant [69]. Future studies evaluating the clinical relevance of changes in LSM on changes in histology and liver-related outcomes are needed.
Table 1.
Studies evaluating longitudinal changes in elastography and changes in histology or liver-related outcomes.
| Author date |
Study design N | Mode of elastography | Median time to follow up | Baseline fibrosis stage | Difference in liver stiffness | Outcome |
|---|---|---|---|---|---|---|
| Petta [64] 2020 | Retrospective longitudinal N = 533 with follow-up LSM |
VCTE | 37 months | F3–F4 or liver stiffness > 10 kPa | ≥20% increase | Liver decompensation: 14.4% of those with a 20% increase compared to 6.2% with a stable reading and 3.8% with >20% decrease |
| Jayakumar [67] 2019 | Prospective longitudinal N = 54 | MRE 2D | 5.6 months | F2–F3 | Any decrease | Fibrosis improvement on liver biopsy 48.0% (any decrease in MRE) vs 20.7% no change in increase in MRE |
| Ajmera [68] 2020 | Prospective longitudinal N = 102 |
MRE 2D | 16.8 month | F0–F1: 62% F2–F4: 38% |
≥15% increase | Fibrosis progression on liver biopsy: 47.1% (≥15% increase) vs 20.0% (not ≥ 15% increase) |
| Gidener [66] 2020 | Prospective longitudinal N = 829 of whom 639 are non-cirrhotic | MRE 2D | 4 years non-cirrhotic cohort 4.4 years cirrhotic cohort |
Non-cirrhotic cohort <3.5 kPa: 74% ≥3.5 kPa: 26% |
Per 1 kPa increase at baseline | Hazard ratio for future cirrhosis development 2.93 per kPa increment. Hazard ratio for future decompensation or death among patients with compensated cirrhosis 1.32 per kPa increment |
6. Conclusion
Despite being the most common chronic liver disease globally, liver biopsy remains the reference standard for staging NAFLD severity. Given the projected increase in the burden of advanced liver disease from NAFLD and emerging therapeutic options, the non-invasive identification and monitoring of this population is a major unmet need. Current imaging biomarkers to diagnose NAFLD and quantify liver fat are accurate and in the case of MRI-PDFF may be more informative than liver biopsy. However, imaging biomarkers for diagnosing NASH remain limited. Elastography can accurately diagnose advanced fibrosis and in the case of MRE provides high diagnostic accuracy for earlier stages of fibrosis. Strategies to combine multiple platforms of biomarkers in specific contexts of use with established cutoff points will be necessary to guide the management of patients with NAFLD. Furthermore, additional multicenter longitudinal studies of the impact of changes in non-invasive imaging biomarkers on histology and liver-related events are needed to minimize relying on liver biopsy in NAFLD.
Funding
R.L. receives funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), DOD PRCRP (W81XWH-18-2-0026), NIDDK (U01DK061734, R01DK106419, R01DK121378, R01DK124318, and P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019).
V.A. is supported by NIDDK (K23DK119460).
Author contributions
Both authors contributed to the study concept and design, drafting the manuscript, critical revisions, and approval of the final manuscript.
Conflict of interest
Potential conflicts of interest for Rohit Loomba: Dr. Loomba serves as a consultant or advisory board member for Bird Rock Bio, Celgene, Enanta, GRI Bio, Madrigal, Metacrine, NGM, Sanofi, Arrowhead Research, Galmed, NGM, GNI, Novo Nordisk, Merck, Siemens, Pfizer, Gilead, and Glympse Bio. His institution has received grant support from Allergan, BMS, BI, Daiichi-Sankyo Inc., Eli Lilly, Galectin, Galmed, GE, Genfit, Intercept, Janssen Inc., Madrigal, Merck, NGM, Pfizer, Prometheus, Siemens, and Sirius. He is also co-founder of Liponexus Inc.
Contributor Information
Veeral Ajmera, Email: V1ajmera@ucsd.edu.
Rohit Loomba, Email: roloomba@ucsd.edu.
References
- 1.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 3.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Stepanova M., Younossi Y., Golabi P., Mishra A., Rafiq N. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69(3):564–568. doi: 10.1136/gutjnl-2019-318813. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. Journal of Hepatology. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. Journal of Hepatology. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 7.Stefan N., Häring H.U., Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinologica. 2019;7(4):313–324. doi: 10.1016/S2213-8587(18)30154-2. [DOI] [PubMed] [Google Scholar]
- 8.Kwong A., Kim W.R., Lake J.R., Smith J.M., Schladt D.P., Skeans M.A. OPTN/SRTR 2018 annual data report: liver. American Journal of Transplantation. 2020;20(Suppl s1):193–299. doi: 10.1111/ajt.15674. [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z., Stepanova M., Ong J.P., Jacobson I.M., Bugianesi E., Duseja A. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clinical Gastroenterology and Hepatology. 2019;17(4):748–755. doi: 10.1016/j.cgh.2018.05.057. e743. [DOI] [PubMed] [Google Scholar]
- 10.Haldar D., Kern B., Hodson J., Armstrong M.J., Adam R., Berlakovich G. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European Liver Transplant Registry study. Journal of Hepatology. 2019;71(2):313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loomba R., Wong R., Fraysse J., Shreay S., Li S., Harrison S. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Alimentary Pharmacology & Therapeutics. 2020;51(11):1149–1159. doi: 10.1111/apt.15679. [DOI] [PubMed] [Google Scholar]
- 12.Ratziu V., Charlotte F., Heurtier A., Gombert S., Giral P., Bruckert E. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 13.Merriman R.B., Ferrell L.D., Patti M.G., Weston S.R., Pabst M.S., Aouizerat B.E. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44(4):874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 14.Hernaez R., Lazo M., Bonekamp S., Kamel I., Brancati F.L., Guallar E. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Wang C., Duanmu Y., Zhang C., Zhao W., Wang L. Comparison of CT and magnetic resonance mDIXON-Quant sequence in the diagnosis of mild hepatic steatosis. British Journal of Radiology. 2018;91(1091):20170587. doi: 10.1259/bjr.20170587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Poorten D., Samer C.F., Ramezani-Moghadam M., Coulter S., Kacevska M., Schrijnders D. Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause? Hepatology. 2013;57(6):2180–2188. doi: 10.1002/hep.26072. [DOI] [PubMed] [Google Scholar]
- 17.Strauss S., Gavish E., Gottlieb P., Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. American Journal of Roentgenology. 2007;189(6):W320–W323. doi: 10.2214/AJR.07.2123. [DOI] [PubMed] [Google Scholar]
- 18.Lin S.C., Heba E., Wolfson T., Ang B., Gamst A., Han A. Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clinical Gastroenterology and Hepatology. 2015;13(7):1337–1345. doi: 10.1016/j.cgh.2014.11.027. e1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han A., Zhang Y.N., Boehringer A.S., Montes V., Andre M.P., Erdman J.W., Jr. Assessment of hepatic steatosis in nonalcoholic fatty liver disease by using quantitative US. Radiology. 2020;295(1):106–113. doi: 10.1148/radiol.2020191152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlas T., Petroff D., Sasso M., Fan J.G., Mi Y.Q., de Ledinghen V. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. Journal of Hepatology. 2017;66(5):1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Caussy C., Alquiraish M.H., Nguyen P., Hernandez C., Cepin S., Fortney L.E. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2018;67(4):1348–1359. doi: 10.1002/hep.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajmera V.H., Cachay E.R., Ramers C.B., Bassirian S., Singh S., Bettencourt R. Optimal threshold of controlled attenuation parameter for detection of HIV-associated NAFLD with magnetic resonance imaging as the reference standard. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa429. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eddowes P.J., Sasso M., Allison M., Tsochatzis E., Anstee Q.M., Sheridan D. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–1730. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 24.Wong V.W.-S., Petta S., Hiriart J.-B., Cammà C., Wong G.L.-H., Marra F. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. Journal of Hepatology. 2017;67(3):577–584. doi: 10.1016/j.jhep.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Caussy C., Brissot J., Singh S., Bassirian S., Hernandez C., Bettencourt R. Prospective, same-day, direct comparison of controlled attenuation parameter with the M vs the XL probe in patients with nonalcoholic fatty liver disease, using magnetic resonance imaging–proton density fat fraction as the standard. Clinical Gastroenterology and Hepatology. 2020;18(8):1842–1850. doi: 10.1016/j.cgh.2019.11.060. e1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeder S.B., Cruite I., Hamilton G., Sirlin C.B. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. Journal of Magnetic Resonance Imaging. 2011;34(4):729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szczepaniak L.S., Nurenberg P., Leonard D., Browning J.D., Reingold J.S., Grundy S. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. American Journal of Physiology Endocrinology and Metabolism. 2005;288(2):E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 28.Doycheva I., Cui J., Nguyen P., Costa E.A., Hooker J., Hofflich H. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Alimentary Pharmacology & Therapeutics. 2016;43(1):83–95. doi: 10.1111/apt.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulai P.S., Sirlin C.B., Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. Journal of Hepatology. 2016;65(5):1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loomba R., Sirlin C.B., Ang B., Bettencourt R., Jain R., Salotti J. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61(4):1239–1250. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Permutt Z., Le T.A., Peterson M.R., Seki E., Brenner D.A., Sirlin C. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Alimentary Pharmacology & Therapeutics. 2012;36(1):22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang A., Desai A., Hamilton G., Wolfson T., Gamst A., Lam J. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274(2):416–425. doi: 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park C.C., Nguyen P., Hernandez C., Bettencourt R., Ramirez K., Fortney L. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152(3):598–607. doi: 10.1053/j.gastro.2016.10.026. e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang G.H., Cruite I., Shiehmorteza M., Wolfson T., Gamst A.C., Hamilton G. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. Journal of Magnetic Resonance Imaging. 2011;34(4):928–934. doi: 10.1002/jmri.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mashhood A., Railkar R., Yokoo T., Levin Y., Clark L., Fox-Bosetti S. Reproducibility of hepatic fat fraction measurement by magnetic resonance imaging. Journal of Magnetic Resonance Imaging. 2013;37(6):1359–1370. doi: 10.1002/jmri.23928. [DOI] [PubMed] [Google Scholar]
- 36.Noureddin M., Lam J., Peterson M.R., Middleton M., Hamilton G., Le T.A. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58(6):1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomba R., Neuschwander-Tetri B.A., Sanyal A., Chalasani N., Diehl A.M., Terrault N. Multicenter validation of association between decline in MRI-PDFF and histologic response in NASH. Hepatology. 2020 doi: 10.1002/hep.31121. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imajo K., Kessoku T., Honda Y., Tomeno W., Ogawa Y., Mawatari H. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626–637. doi: 10.1053/j.gastro.2015.11.048. e627. [DOI] [PubMed] [Google Scholar]
- 39.Ajmera V., Park C.C., Caussy C., Singh S., Hernandez C., Bettencourt R. Magnetic resonance imaging proton density fat fraction associates with progression of fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155(2):307–310. doi: 10.1053/j.gastro.2018.04.014. e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui J., Philo L., Nguyen P., Hofflich H., Hernandez C., Bettencourt R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. Journal of Hepatology. 2016;65(2):369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le T.A., Chen J., Changchien C., Peterson M.R., Kono Y., Patton H. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56(3):922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caussy C., Reeder S.B., Sirlin C.B., Loomba R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 2018;68(2):763–772. doi: 10.1002/hep.29797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stine J.G., Munaganuru N., Barnard A., Wang J.L., Kaulback K., Argo C.K. Change in MRI-PDFF and histologic response in patients with nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology. 2020 doi: 10.1016/j.cgh.2020.08.061. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ajmera V., Loomba R. Can elastography differentiate isolated fatty liver from nonalcoholic steatohepatitis? Seminars in Liver Disease. 2018;38(1):14–20. doi: 10.1055/s-0037-1618587. [DOI] [PubMed] [Google Scholar]
- 45.Pavlides M., Banerjee R., Tunnicliffe E.M., Kelly C., Collier J., Wang L.M. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver International. 2017:1065–1073. doi: 10.1111/liv.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jayaswal A.N.A., Levick C., Selvaraj E.A., Dennis A., Booth J.C., Collier J. Prognostic value of multiparametric magnetic resonance imaging, transient elastography and blood-based fibrosis markers in patients with chronic liver disease. Liver International. 2020 doi: 10.1111/liv.14625. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Loomba R., Wolfson T., Ang B., Hooker J., Behling C., Peterson M. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60(6):1920–1928. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loomba R., Cui J., Wolfson T., Haufe W., Hooker J., Szeverenyi N. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study. American Journal of Gastroenterology. 2016;111(7):986–994. doi: 10.1038/ajg.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen A.M., Shah V.H., Therneau T.M., Venkatesh S.K., Mounajjed T., Larson J.J. The role of three-dimensional magnetic resonance elastography in the diagnosis of nonalcoholic steatohepatitis in obese patients undergoing bariatric surgery. Hepatology. 2020;71(2):510–521. doi: 10.1002/hep.30483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee H.W., Park S.Y., Kim S.U., Jang J.Y., Park H., Kim J.K. Discrimination of nonalcoholic steatohepatitis using transient elastography in patients with nonalcoholic fatty liver disease. PloS One. 2016;11(6) doi: 10.1371/journal.pone.0157358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cassinotto C., Boursier J., de Ledinghen V., Lebigot J., Lapuyade B., Cales P. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63(6):1817–1827. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 52.Herrmann E., De Lédinghen V., Cassinotto C., Chu W.C.-W., Leung V.Y.-F., Ferraioli G. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: an individual patient data-based meta-analysis. Hepatology. 2018;67(1):260–272. doi: 10.1002/hep.29179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong V.W., Vergniol J., Wong G.L., Foucher J., Chan A.W., Chermak F. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. American Journal of Gastroenterology. 2012;107(12):1862–1871. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 54.Castera L., Foucher J., Bernard P.H., Carvalho F., Allaix D., Merrouche W. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51(3):828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 55.Siddiqui M.S., Vuppalanchi R., Van Natta M.L., Hallinan E., Kowdley K.V., Abdelmalek M. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology. 2019;17(1):156–163. doi: 10.1016/j.cgh.2018.04.043. e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu C., Caussy C., Imajo K., Chen J., Singh S., Kaulback K. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clinical Gastroenterology and Hepatology. 2019;17(4):630–637. doi: 10.1016/j.cgh.2018.05.059. e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner M., Corcuera-Solano I., Lo G., Esses S., Liao J., Besa C. Technical failure of MR elastography examinations of the liver: experience from a large single-center study. Radiology. 2017;284(2):401–412. doi: 10.1148/radiol.2016160863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh S., Venkatesh S.K., Loomba R., Wang Z., Sirlin C., Chen J. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. European Radiology. 2016;26(5):1431–1440. doi: 10.1007/s00330-015-3949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh S., Venkatesh S.K., Wang Z., Miller F.H., Motosugi U., Low R.N. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clinical Gastroenterology and Hepatology. 2015;13(3):440–451. doi: 10.1016/j.cgh.2014.09.046. e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J., Yin M., Talwalkar J.A., Oudry J., Glaser K.J., Smyrk T.C. Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology. 2017;283(2):418–428. doi: 10.1148/radiol.2016160685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vilar-Gomez E., Lou Z., Kong N., Vuppalanchi R., Imperiale T.F., Chalasani N. Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Clinical Gastroenterology and Hepatology. 2020;18(10):2305–2314. doi: 10.1016/j.cgh.2020.04.017. e2312. [DOI] [PubMed] [Google Scholar]
- 62.Newsome P.N., Sasso M., Deeks J.J., Paredes A., Boursier J., Chan W.K. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterology and Hepatology. 2020;5(4):362–373. doi: 10.1016/S2468-1253(19)30383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petta S., Wong V.W.-S., Cammà C., Hiriart J.-B., Wong G.L.-H., Vergniol J. Serial combination of non-invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Alimentary Pharmacology & Therapeutics. 2017;46(6):617–627. doi: 10.1111/apt.14219. [DOI] [PubMed] [Google Scholar]
- 64.Shili-Masmoudi S., Wong G.L.H., Hiriart J.B., Liu K., Chermak F., Shu S.S.T. Liver stiffness measurement predicts long-term survival and complications in non-alcoholic fatty liver disease. Liver International. 2020;40(3):581–589. doi: 10.1111/liv.14301. [DOI] [PubMed] [Google Scholar]
- 65.Petta S., Sebastiani G., Viganò M., Ampuero J., Wai-Sun Wong V., Boursier J. Monitoring occurrence of liver-related events and survival by transient elastography in patients with nonalcoholic fatty liver disease and compensated advanced chronic liver disease. Clinical Gastroenterology and Hepatology. 2020 doi: 10.1016/j.cgh.2020.06.045. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Han M.A.T., Vipani A., Noureddin N., Ramirez K., Gornbein J., Saouaf R. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: a multicenter study. Liver International. 2020;40(9):2242–2251. doi: 10.1111/liv.14593. [DOI] [PubMed] [Google Scholar]
- 67.Gidener T., Ahmed O.T., Larson J.J., Mara K.C., Therneau T.M., Venkatesh S.K. Liver stiffness by magnetic resonance elastography predicts future cirrhosis, decompensation and death in NAFLD. Clinical Gastroenterology and Hepatology. 2020 doi: 10.1016/j.cgh.2020.09.044. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jayakumar S., Middleton M.S., Lawitz E.J., Mantry P.S., Caldwell S.H., Arnold H. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: analysis of data from a phase II trial of selonsertib. Journal of Hepatology. 2019;70(1):133–141. doi: 10.1016/j.jhep.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 69.Ajmera V.H., Liu A., Singh S., Yachoa G., Ramey M., Bhargava M. Clinical utility of an increase in magnetic resonance elastography in predicting fibrosis progression in nonalcoholic fatty liver disease. Hepatology. 2020;71(3):849–860. doi: 10.1002/hep.30974. [DOI] [PMC free article] [PubMed] [Google Scholar]