Abstract
Cytokines are important immunotherapeutics with approved drugs for the treatment of human cancers. However, systemic administration of cytokines often fails to achieve adequate concentrations to immune cells in tumors due to dose-limiting toxicity. Thus, developing localized therapy that directly delivers immune-stimulatory cytokines to tumors may improve the therapeutic efficacy. In this study, we generated novel lipid nanoparticles (LNPs) encapsulated with mRNAs encoding cytokines including IL-12, IL-27 and GM-CSF, and tested their anti-tumor activity. We first synthesized ionizable lipid materials containing di-amino groups with various head groups (DALs). The novel DAL4-LNP effectively delivered different mRNAs in vitro to tumor cells and in vivo to tumors. Intratumoral injection of DAL4-LNP loaded with IL-12 mRNA was most potent in inhibiting B16F10 melanoma tumor growth compared to IL-27 or GM-CSF mRNAs in monotherapy. Furthermore, intratumoral injection of dual DAL4-LNP-IL-12 mRNA and IL-27 mRNA showed a synergistic effect in suppressing tumor growth without causing systematic toxicity. Most importantly, intratumoral delivery of IL-12 and IL-27 mRNAs induced robust infiltration of immune effector cells, including IFN-γ and TNF-α producing NK and CD8+ T cells into tumors. Thus, intratumoral administration of DAL-LNP loaded with IL-12 and IL-27 mRNA provides a new treatment strategy for cancer.
Keywords: Diamino lipid-derived nanoparticles (DAL-LNPs), cytokines, mRNA therapeutics, cancer immunotherapy
Graphical Abstract
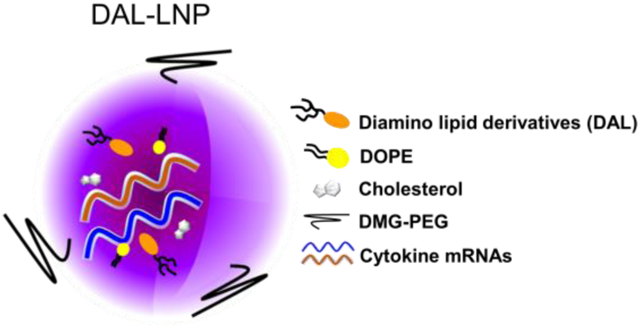
Introduction
Cytokines are one of the first immunotherapeutics applied for treating human cancers. In 1986, IFN-α was first approved by the FDA for the treatment of hairy cell leukemia1. IL-2 was later approved for metastatic renal cell cancer and advanced melanoma in 1992 and 1998, respectively2. Currently, many cytokines are explored in clinical trials, including IL-12, IL-15, IL-21, and GM-CSF3. Despite efforts to develop systemic cytokine monotherapy for cancers, the delivery of cytokines hinders the initial excitement due to a narrow therapeutic margin. Cytokines act in a paracrine or autocrine fashion and have a relatively short half-life. Therefore, large quantities of cytokines must be systemically administered to achieve a sufficient concentration within the tumor microenvironment (TME), which is often associated with severe toxicities4–6. Due to dose-limiting toxicities, lower doses of cytokines are often used in clinical trials, which result in low concentrations of the cytokines in the TME. Thus, developing localized therapy that directly delivers immune-stimulatory cytokines to tumors may be a promising strategy to overcome this dilemma.
IL-12, IL-27, and GM-CSF have shown dynamic anti-tumor activities in various animal models. IL-12 demonstrates potent anti-tumor activity7 via enhanced Th1/Tc1 response7, 8 and T cell recruitment to tumors9. However, systemically delivered recombinant IL-12 can lead to fatal consequences5, 6. IL-12-induced systemic toxicity can only be relieved if IL-12 expression is confined to the tumor site17. To avoid systemic toxicity, researchers have designed various strategies10–16 to _target or restrict IL-12 to tumors. IL-27 is also an anti-inflammatory cytokine that exhibits robust anti-tumor effects. Preclinical models in mice have indicated that both endogenous18–21 and exogenous22–24 IL-27 inhibits tumor growth in vivo. IL-27 enhances the T cell survival in TME and promotes the generation of memory T cells by programming CD8+ T cells into a unique T effector phenotype, characterized by increased secretion of IFN-γ and IL-1022, 25. Likewise, prior studies found that mice inoculated with live or irradiated tumor cells expressing high levels of GM-CSF (GVAX) resulted in the recruitment of antigen-presenting cells such as dendritic cells in tumor sites, which led to induction of anti-tumor immunity and tumor rejection26, 27. The efficacy of autologous and allogeneic GVAX was later reported either as a single drug or in combination with other immunomodulators in various animal models and clinical studies28–30. GM-CSF has also been included in two clinically approved anti-tumor vaccines, i.e. Sipuleucel-T31 and T-VEC32. Systemically delivered GM-CSF has shown potential clinical benefits including reduced toxicity when used in combination with ipilimumab33, 34 35.
Potent induction of anti-tumor immunity impeded by systemic toxicity of cytokines strongly justifies the development of clinically relevant, cytokine-based localized therapy. Recently, we found that intratumoral delivery of AAV-IL-27 could repress tumor growth and induce anti-tumor immunity36. However, AAV-mediated IL-27 delivery poses potential pitfalls as there is no termination of IL-27 production when the biological activity of IL-27 is sufficient. Therefore, novel approaches that can adeptly deliver immunostimulatory cytokines to tumors are necessary. In this context, we consider that using lipid nanoparticles to deliver mRNAs of immune stimuli to TME is a highly feasible approach. LNPs possess unique features including (i) easy preparation and chemical synthesis for large-scale production; (ii) efficient encapsulation and delivery of mRNA; (iii) transient, mRNA-induced expression of protein; and (iv) low potential systemic toxicity via intratumoral administration.
In this study, we examined the local administration of LNPs encapsulating cytokine mRNA combinations that have not yet been tested in a mouse melanoma model. Intratumoral administration of IL-12 and IL-27 mRNAs by DAL-LNP promoted sustained inhibition of B16F10 melanoma growth without causing significant toxicity. Mechanistically, robust infiltration of immune effector cells, including NK and CD8+ T cells, into tumor tissues was observed after intratumoral delivery of IL-12 and IL-27 mRNA. In summary, we discovered a new mRNA delivery LNPs formulation and a cytokine combination that can be used to expand current cancer treatments. Such delivery platform merits further development as a novel cancer therapeutic.
Materials and Methods
Reagents
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise noted. DOPE was purchased from Avanti Polar Lipids, Inc (Alabaster, AL, USA) and DMG-PEG2000 was purchased from NOF America Corporation (White Plains, NY, USA).
Synthesis of DALs
Compounds b, c and DAL1 were synthesized according to methods reported previously37.
Synthesis of DAL2: DAL1 (250 mg, 0.35 mmol) was first hydrolyzed by NaOH aqueous (1 M) in a mixture of THF and MeOH at 70 °C for 3 hrs. Next, 100 mL CH2Cl2 was added, which was dried with MgSO4. Then, the solvent was evaporated and 10 mL anhydrous THF was added to the residue. NHS (125 mg, 1.05 mmole) and DCC (230 mg, 1.05 mmole) were added to the solution. The resulting mixture was stirred at room temperature overnight. Tert-butylamine (80 mg, 1.05 mmole) and TEA (150 μL, 1.2 mmole) were added to the above reaction mixture. The resulting mixture was stirred at room temperature overnight. After the solvent was removed under reduced temperature, the residue was purified by column chromatography using a CombiFlash Rf system with a RediSep Gold Resolution silica column (Teledyne Isco) with gradient elution (CH2Cl2 and ultra) from 100% CH2Cl2 to 80% CH2Cl2 (ultra, CH2Cl2/MeOH/NH4OH =75/22/3 by volume) to generate 96 mg of oil like DAL2. Using different head groups, DAL3-7 were synthesized following the same procedure as used for the synthesis of DAL2. The amine used for synthesizing DAL4 was 5-Fluoro-2-aminomethylphenylboronic acid, pinacol ester. The pinacol ester was hydrolyzed during purification through column chromatography to yield pure DAL4. The 1H NMR spectra of DAL2–7 are provided in Supplementary Fig. 1.
mRNA synthesis
IL-12, IL-27, and GM-CSF plasmids were purchased from InvivoGen (San Diego, CA, USA) and were amplified to generate templates for in vitro transcription. DNA sequences of the cytokines used in this study are listed in supplementary data. mRNA transcripts were synthesized as reported previously37, 38. mRNAs were synthesized with full substitution of UTP by pseudouridine-5’-triphosphate (TriLink, USA) using AmpliScribe T7-Flash Transcription Kit (Lucigen, USA) following the manufacturer’s instruction. The resulting mRNAs were then purified by RNA Clean & Concentrator (Zymo, USA) and capped using Vaccinia Capping System (NEB, USA) and Cap 2´-O-Methyltransferase (NEB, USA). After one final round of purification, mRNA concentrations were measured using a NanoDrop 2000 Spectrophotometer (ThermoFisher, USA) and stored at −80°C for future use.
Preparation and characterization of LNPs-mRNA
LNPs-mRNA were prepared by mixing lipid materials dissolved in ethanol and mRNA diluted in 10 mM citrate buffer, pH=339. The molar ratio of MC3 LNPs was MC3: DSPC: Cholesterol: DMG-PEG=50:10:38.5:1.5. For DAL-LNPs, the molar ratio was DALs: DOPE: Cholesterol: DMG-PEG=20:30:40:0.75. LNPs-mRNA were prepared by rapidly blending the aqueous and ethanol phases with a pipette for in vitro studies. LNPs-mRNA were prepared by a microfluidic device for in vivo studies (Precision NanoSystems, Vancouver, BC, Canada) and dialyzed in PBS (Slide-A-Lyzer™ Dialysis Cassettes, 3.5K MWCO, ThermoFisher, USA). The parameters of microfluidic device: flow rate: total 12mL/min, aqueous phase/ethanol phase = 3/1, room temperature. The size and zeta potential of DAL-LNPs were measured by Zetasizer (Malvern, USA). The mRNA encapsulation efficiency (EE%) was determined by the RiboGreen assay (Invitrogen, USA). Cryo-EM image of DAL4-LNP-IL-12+IL-27 was obtained by Glacios Cryo Transmission Electron Microscope (ThermoFisher, USA) using a similar method reported before37.
In vitro delivery of cytokine mRNAs LNP to B16F10 melanoma cells
B16F10 cells were originally purchased from ATCC and were maintained in RPMI 1640 Medium (Corning) with fetal bovine serum 10% (v/v). To quantify the delivery efficacy and production of mRNA encoded cytokines in MC3-LNP or DAL4-LNP, we treated the cultured B16F10 cells in a 96 well plate with MC3-IL-12/IL-27/GM-CSF, or DAL4-LNP-IL-12/IL-27/GM-CSF with a dose of 50 ng mRNA of each cytokine mRNA. After 18 hrs incubation, the supernatants of cell cultures were collected, and respective cytokines were quantified by ELISA following standard procedures.
In vivo anti-cancer efficacy of cytokine mRNA LNPs
All animal experiments were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of The Ohio State University and were approved by the Animal Ethics Committee of Institutional Animal Care and Use Committee (IACUC). To establish the mice tumor model, 1 × 105 B16F10 cells in 100 μL PBS were subcutaneously injected in female C57BL/6 mice. Mice were randomly grouped (n = 5–7 in each group) when the tumor reached about 50 mm3. Tumor volume is calculated by length × (width)2/2. For the biodistribution study, intratumoral injection of GFP mRNA in DAL4-LNP (10 μg mRNA/injection) was performed one-time. GFP expression in the tumor microenvironment was analyzed at 24 hrs. For treatment efficacy studies, intratumoral injections of cytokine mRNA LNPs (2 μg mRNA/injection in Fig. 4; 2 μg IL-12 mRNA/injection or 6 μg IL-27 mRNA/injection in Supplementary Fig. 2) were performed every other day for six doses in total. For toxicity studies, intratumoral treatment with cytokine mRNA LNPs (2 μg mRNA/injection) was performed every other day for three times in total. The tumor size was checked every other day. The body weight was checked on days 8, 12, and 14 after tumor inoculation. Paraffin-embedded tissues (heart, lung, liver and kidney) were prepared from DAL4-LNP-IL-12 + IL-27 mRNA treated mice (n=5) or control-treated mice (n=5). Hematoxylin and eosin (H.E.) stained slides were evaluated under a microscope. Representative images were photographed and are provided in Supplementary Fig 4.
Fig. 4. In vivo anti-cancer activity of DAL4-LNP encapsulating single or multiple cytokine mRNAs.
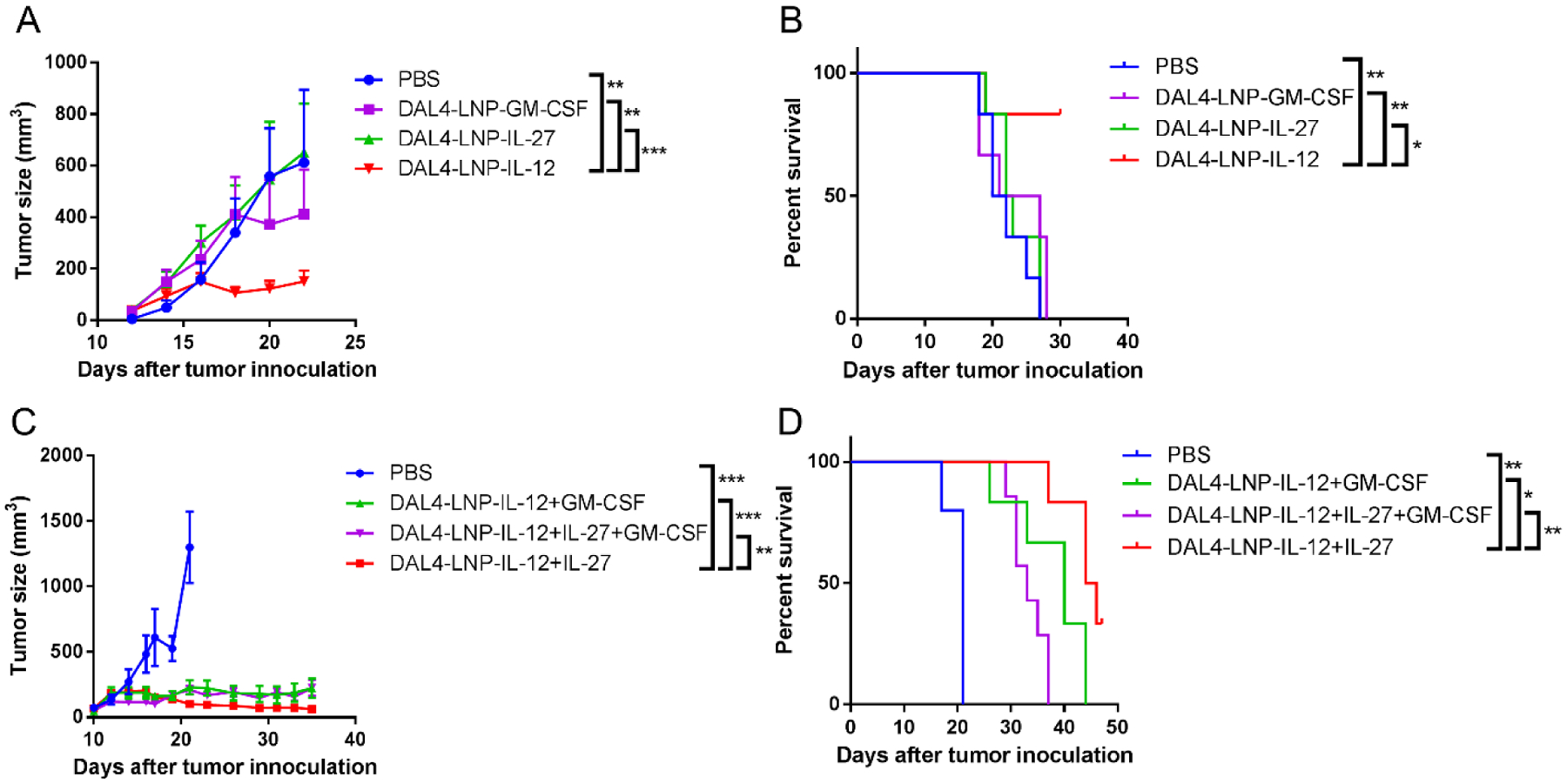
(A) B16F10 tumor size after treatment of single cytokine mRNA in DAL4-LNP (n = 6). (B) Overall survival of B16F10 tumor-bearing mice. (C) B16F10 tumor size after treatment with DAL4-LNP encapsulating two or three cytokine mRNAs (n = 5–7). (D) Overall survival of B16F10 tumor-bearing mice. Data in (A) and (C) are presented as the mean ± S.E.M. Statistical significance in (A) and (C) was analyzed using two-way ANOVA with repeated measurements. Statistical significance in (B), (D) were analyzed using the log-rank (Mantel-Cox) test. *P < 0.05; **P < 0.01; ***P < 0.001.
Antibodies and flow cytometry
Fluorescence labeled monoclonal antibodies to mouse CD45 (30-F11), CD11b (M1/70), F4/80 (745–2342), NK1.1 (Pk136), CD3 (145–2c11), CD4 (GK1.5), CD8α (53–6.7), CD19 (1D3), IFN-γ (XMG1.2), TNF-α (XT22), and isotype control antibodies were purchased from BD Biosciences or Biolegend. Mononuclear cells from tumors were prepared as described40, 41. For staining cell surface markers, cells were incubated with antibodies in staining buffer (0.1 M PBS, 1% FCS and 0.1% sodium azide) on ice for 30 minutes. Cells were washed three times after staining and fixed in 1% paraformaldehyde. For intracellular staining of IFN-γ and TNF-α, cells were first stimulated with cell stimulation cocktail (Invitrogen, USA) for 4 hrs. The cells were then stained for the cell surface markers (CD3/4/8/NK1.1) followed by a standard intracellular cytokine staining procedure. Stained cells were harvested using a Celesta flow cytometer (BD) and data was analyzed using FlowJo software (Tree Star, Inc., OR).
Statistical analysis
Mann-Whitney test and one-way ANOVA with Dunnett’s multiple comparisons were used to analyze in vitro data (GraphPad Prism, CA, USA); Two-way ANOVA with repeated measurements was used to analyze tumor volume and body weight data (R3.4.3, The R Foundation); the survival of tumor-bearing mice was analyzed using log-rank tests (GraphPad Prism). All tests were two-tailed and P < 0.05 was considered statistically significant.
Results
Synthesis of diamino lipid derivatives
We first designed and synthesized a library of seven di-amino lipid materials (DALs) with the same diamino core and carbon chains with different head groups (Fig. 1A). These DAL compounds provide beneficial characteristics. For example, various functional groups can be installed on the phenyl ring such as boronic acid, hydroxyl group, and halogen. These groups enable us to fine-tune several physicochemical properties such as size, hydrophobicity, and charge of the lipids and their corresponding nanoparticles. Fig. 1B displays a representative synthetic route. Compound a underwent a substitution reaction with b to yield intermediate c. Subsequently, a reductive amination reaction between c and d produced DAL137. Removal of ethyl group on DAL1 formed e, which was further reacted with 5-Fluoro-2-aminomethylphenylboronic acid, pinacol ester and hydrolyzed to synthesize DAL4. We confirmed the structures of all these DALs using both 1H NMR and mass spectrum (MS) (Supplementary Data and Supplementary Fig. 1).
Fig. 1. Synthesis of diamino lipid derivatives (DALs).
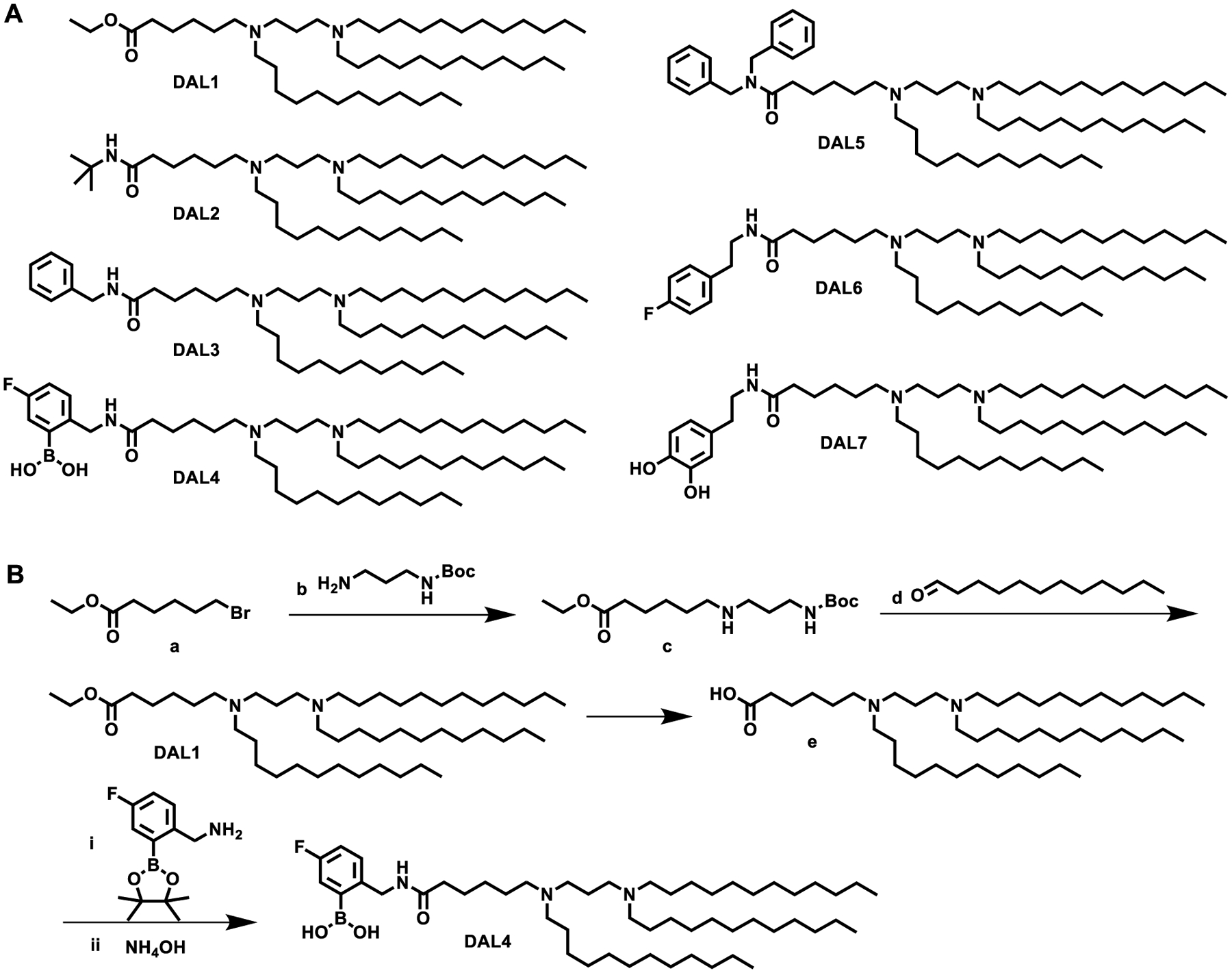
(A) The structures of DALs. (B) Synthetic routes of DAL1 and DAL4.
Formulations of DAL lipid nanoparticles (DAL-LNPs) for in vitro mRNA delivery and cytokine expressions
Next, we studied the mRNA delivery efficiency of DAL lipid nanoparticles (DAL-LNPs) using luciferase mRNA in B16F10 melanoma tumor cells in vitro. We formulated DALs with 1,2-dioleoylsnglycero-3-phosphoethanolamine (DOPE), cholesterol (Chol), 1,2-dimyristoyl-racglycero-3-methylpolyoxyethylene (DMG-PEG2000, PEG) (molar ratio: DAL/DOPE/Chol/PEG = 20/30/40/0.75), and luciferase mRNA to prepare the nanoparticles (DAL-LNP-Luc) as described previously37. The mRNA encapsulation efficiency (Fig. 2A), formulation size distribution, (Fig. 2B) and polydispersity index (PDI) (Fig. 2B) were also determined. The mRNA encapsulation efficiency of DAL-LNPs ranged from 2.6% in DAL1-LNP-Luc to 90.3% in DAL4-LNP-Luc (Fig. 2A). These nanoparticles had a size of 150–200 nm and PDI less than 0.2 as measured by dynamic light scattering (Fig. 2B). After treating the B16F10 cells with DAL-LNPs for 18 hours, the luciferase mRNA delivery efficiency was quantified by a bioluminescence reporter assay. As shown in Fig. 2C, DAL4-LNP induced the highest luminescence signal in cultured cells when compared with other DAL-LNPs and Lipofectamine™ 3000. DAL4-LNP displayed a spherical morphology when visualized by cryo-EM microscopy (Fig. 2D). Thus, we chose DAL4-LNP as a lead candidate to encapsulate cytokine mRNAs for further testing.
Fig. 2. DAL-LNPs delivery of mRNA in vitro.
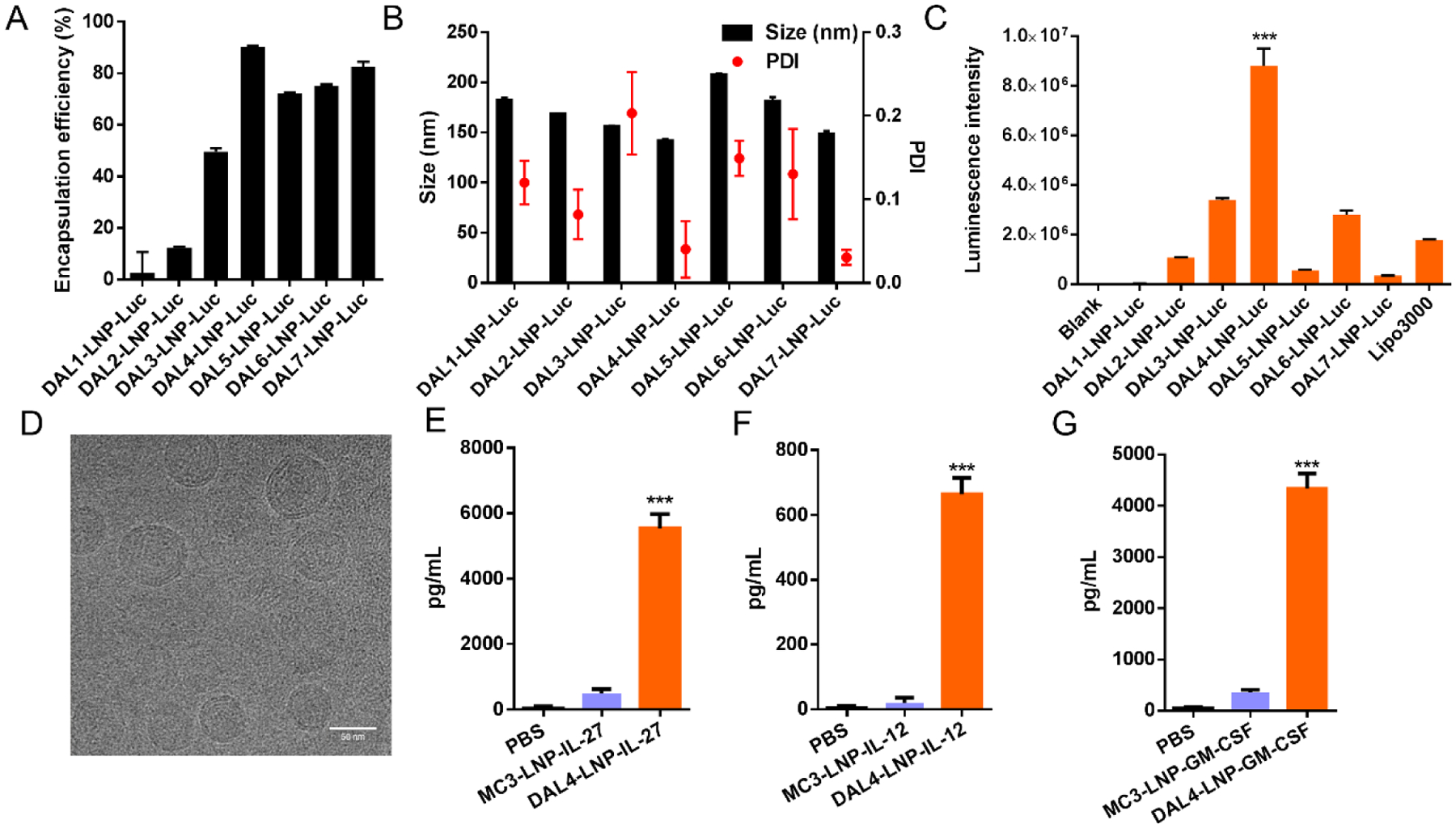
(A) Encapsulation efficiency, (B) size distribution and polydispersity index (PDI) of DAL-LNPs encapsulating luciferase mRNA (DAL-LNP-Luc). (C) In vitro delivery of DAL-LNP-Luc in B16F10 cells. N=3. (D) Cryo-EM image of DAL4-LNP co-encapsulating IL-12 mRNA and IL-27 mRNA. Bar = 50 nm. (E-G) In vitro delivery of MC3-LNP or DAL4-LNP encapsulating either IL-27 mRNA (E), IL-12 mRNA (F), or GM-CSF mRNA (G) in B16F10 cells. N=3–4. The concentrations of cytokines in the supernatants were determined by ELISA. All data are presented as the mean ± S.D. Statistical significance in C, E, F, and G were analyzed using one-way ANOVA with Dunnett’s multiple comparisons test. ***P < 0.001.
We investigated the cytokine mRNA delivery and protein expression in vitro by an ELISA assay. We treated B16F10 cells with DAL4-LNP encapsulating either IL-27 mRNA (DAL4-LNP-IL-27), IL-12 mRNA (DAL4-LNP-IL-12), or GM-CSF mRNA (DAL4-LNP-GM-CSF) for 18 hours and collected culture supernatants. The results (Fig. 2E–G) showed that each of the cytokine proteins can be expressed and secreted in the supernatants of cell culture, which was more efficient than MC3 formulated LNPs, an FDA approved LNP formulation. These results indicated that the DAL4-LNP can effectively deliver cytokine mRNA in cells.
DAL4-LNP can effectively deliver mRNA into tumors for protein expression
After selecting DAL4-LNP from in vitro assays, we tested if DAL4-LNP could be used to deliver mRNA to established tumors for protein expression in vivo. For this purpose, we generated DAL4-LNP loaded with mRNA encoding GFP and i.t. injected DAL4-LNP-GFP to established B16F10 tumors. 12 hours after injection of LNP-GFP mRNA, GFP+ cells were readily detected in tumors, ranging from 3–15% of total tumor cells by flow cytometry (Fig. 3A). Among GFP+ cells, approximately 66.7% were CD45‒ non-immune cells which are primarily tumor cells and fibroblasts, and 31.9% of cells were CD45+ immune cells (Fig. 3B). Among GFP+ immune cells, about 98% were CD19+ B cells, and 2% were monocyte or macrophages, while GFP+ T cells were barely detected (Fig. 3B). Thus, DAL4-LNP can serve as an efficient vehicle for mRNA delivery to tumors for protein expression.
Fig. 3. DAL4-LNP for mRNA delivery to tumors.
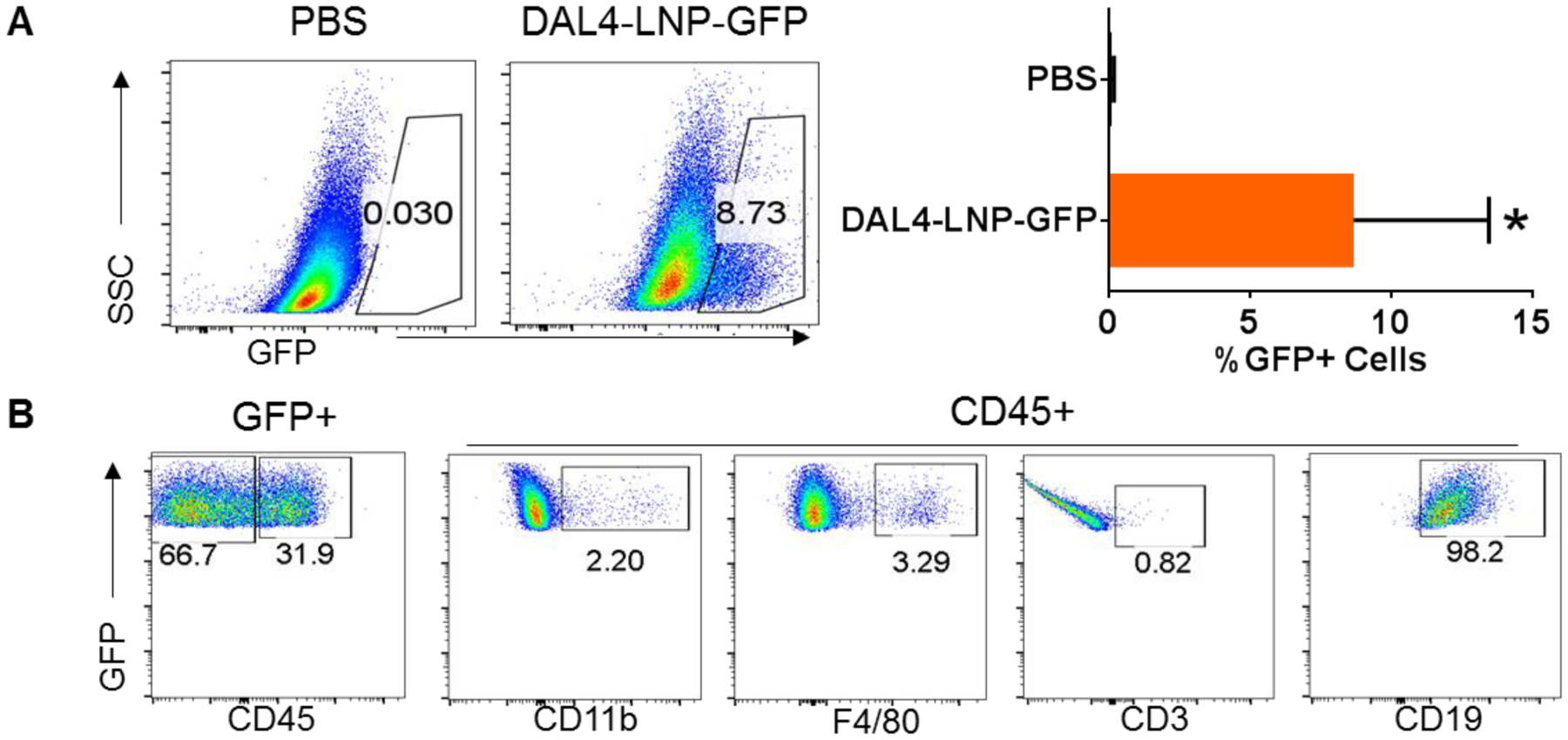
Flow cytometry was used for quantifying GFP+ cells (A) and their subtypes (B). *P<0.05 by Mann-Whitney test.
Intratumoral delivery of LNP-IL-12/IL-27 inhibits tumor growth
Cytokines such as IL-127, IL-2722–24 and GM-CSF26, 27 have demonstrated anti-tumor activity when delivered systemically or locally. To compare the therapeutic potential of each cytokine mRNA in DAL4-LNP, we first probed the treatment efficacy in a subcutaneous B16F10 mouse tumor model by delivering a single cytokine mRNA with DAL4-LNP via intratumoral injection every other day for six doses. DAL4-LNP loaded with IL-12 mRNA (DAL4-LNP-IL-12) exhibited the strongest tumor inhibitive effect when compared to DAL4-LNP encapsulated with IL-27 mRNA (DAL4-LNP-IL-27) or GM-CSF mRNA (DAL4-LNP-GM-CSF) with slower tumor growth and prolonged survival (Fig. 4A–B).
Previous gene therapy based on the systemic application of IL-27 and IL-12 has shown significant synergy of these two cytokines in tumor inhibition42. To determine if intratumoral injections of cytokine mRNA nanoparticles could induce stronger tumor growth inhibition, we tested IL-12 mRNA in combination with IL-27 and/or GM-CSF mRNA in DAL4-LNP. DAL4-LNP-IL-12+IL-27 outperformed other combinations by retarding tumor growth (Fig. 4C) and extending the survival of tumor-bearing mice (Fig. 4D). Interestingly, the combination of all three cytokine mRNAs did not induce better tumor inhibition or prolong survival compared to the IL-12+IL-27 combination. To further calibrate the dosage of DAL4-LNP-IL-12+IL-27, we changed the IL-27 mRNA dose from 2 μg to 6 μg per mouse for six doses while the IL-12 mRNA dose remained at 2 μg (Supplementary Fig. 2). We observed that the increased dosage of DAL4-LNP-IL-27 also showed significant tumor inhibition compared to the control group, and DAL4-LNP-IL-12+IL-27 resulted in 100% survival on day 35. To assess potential systemic toxicity, we comprehensively monitored body weight changes in addition to overall survival during the treatment process and analyzed histology of major organs after DAL4-LNP treatments. No significant body weight changes were observed as compared with control mice (Supplementary Fig. 3). Histology analysis of major organs (heart, lung, liver and kidney) from five treated mice demonstrated no observed inflammatory changes (Supplementary Fig. 4) as compared to control mice. Consequently, intratumoral delivery of DAL4-LNP-IL-12+IL-27 mRNA did not cause noticeable systematic toxicity within the dosages tested.
Intratumoral delivery of DAL4-LNP-IL-12 + IL-27 mRNA induces robust infiltration of immune effector cells into tumors
Both IL-12 and IL-27 have been shown to be potent cytokines capable of inducing T and NK cell responses in tumor models7, 22–24. We hypothesized that intratumoral injection of DAL4-LNP-IL-12 + IL-27 mRNA can induce potent immune responses that lead to the infiltration of immune effector cells into tumors. To test this hypothesis, we analyzed tumors from mice receiving the treatment regimen and examined the density of immune cells in tumors by flow cytometry. DAL4-LNP-IL-12 + IL-27 mRNA-treated tumors resulted in a nearly 10-fold increase of total CD45+ leukocytes compared to control-treated tumors (Fig. 5A). The percentages of almost every subtype of leukocytes among the total tumor mononuclear cells, including CD19+ B cells, F4/80+ macrophages, CD4+ T cells, CD8+ T cells, and NK cells, increased when compared to the control group. Additionally, tumor-infiltrating T and NK cells from DAL4-LNP-IL-12 + IL-27 mRNA treated tumors significantly increased the number of IFN-γ (Fig. 5B) and TNF-α (Fig. 5C) producing cells. Thus, intratumoral delivery of DAL4-LNP-IL-12+IL-27 mRNA induced robust infiltration of immune effector cells into tumors.
Fig. 5. Intratumoral injection of DAL4-LNP-IL-12 + IL-27 mRNA induces robust infiltration of immune effector cells into tumors and stimulates the production of anti-tumor signaling molecules.
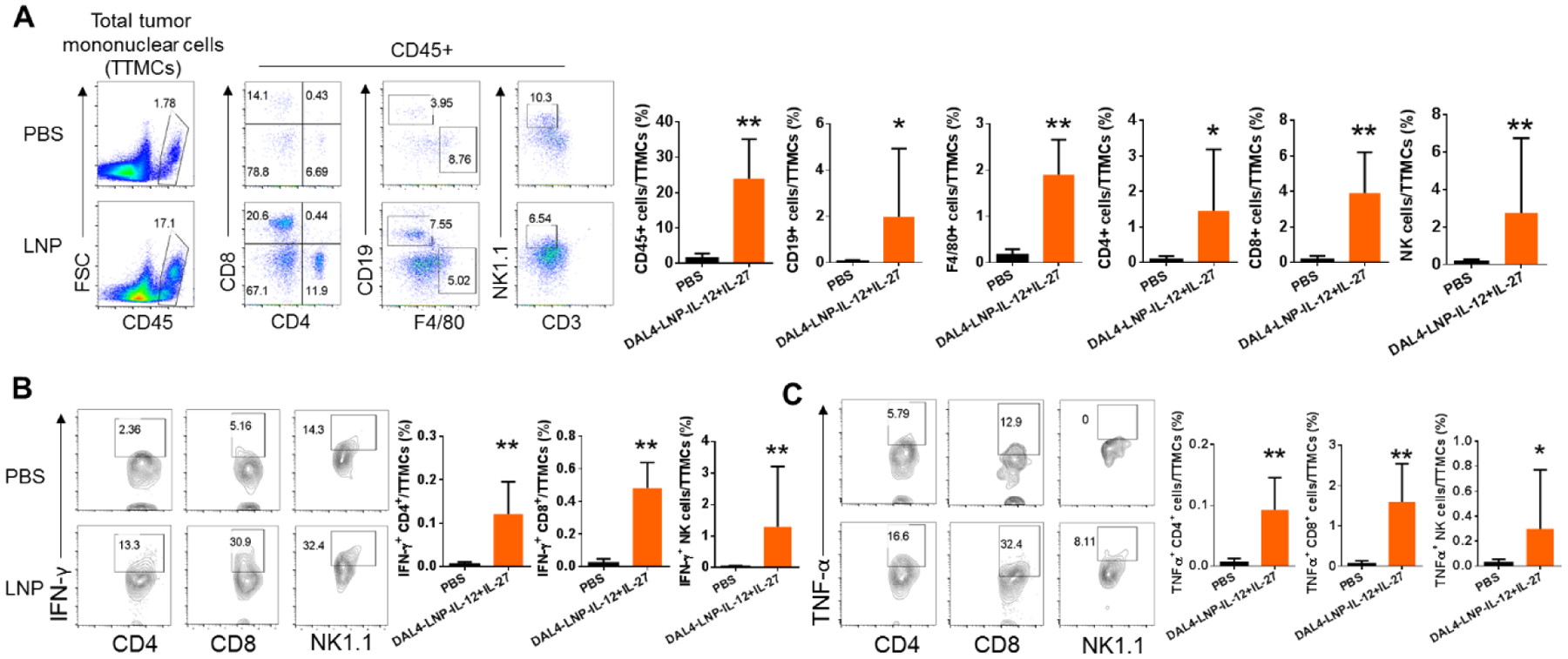
(A) Flow cytometry was used for quantifying CD45+ leukocytes and its subtypes. (B-C) Intracellular staining and flow cytometry were used for quantifying IFN-γ (B) and TNF-α (C) producing T and NK cells. *P<0.05, **P<0.01 by Mann-Whitney tests.
Discussion
In this study, we tested the strategy of using novel lipid nanoparticles (LNPs) to deliver cytokine mRNAs to TME. Our results suggested that DAL4-LNP can efficiently deliver mRNAs both in vitro and in vivo for cytokine expressions. Additionally, intratumoral delivery of dual IL-12 + IL-27 mRNAs demonstrated the most potent inhibition of tumor growth, which was reflected by enhanced infiltration of immune effectors into the tumor vasculature and activation of effector cells to secrete downstream anti-tumor signaling molecules.
Based on the interesting chemical structures of diamines, we first synthesized seven new ionizable lipids containing di-amino groups and various head groups. Next, we formulated these ionizable lipids with phospholipid, cholesterol, and PEG-lipid to encapsulate luciferase mRNA or single or multiple cytokine mRNAs. The DAL-LNP-mRNA used in this study was prepared as previously reported43, 44. The electrostatic complexation between mRNAs and LNPs was ensured by the charge interactions between the negatively charged phosphate groups in mRNA backbones and the protonated amine groups in ionizable DAL lipids. We screened and characterized the mRNA formulation by delivering luciferase mRNA in B16F10 melanoma cells in vitro. DAL4-LNP showed the highest luciferase mRNA delivery efficiency with a size of ~130 nm and mRNA encapsulation efficiency of around 90%. By comparing different DALs based LNPs used in this study, there is no obvious correlation between the properties of LNPs, including size, PDI, or encapsulation efficiency (Fig. 2A, B), and their mRNA delivery efficiency (Fig. 2C). We speculate that the head group of DAL4 might facilitate particle formulation and its interactions with cell and endosome membranes43, 45. However, the mechanism needs to be further uncovered.
The in vivo delivery experiments suggest that DAL4-LNP mRNA delivered by intratumoral injection is an efficient approach for protein expression in mouse tumors. The results showed that 12 hrs after DAL4-LNP-GFP mRNA injection, up to 15% of total tumor cells were GFP+. Among GFP+ cells, about 70% were CD45− cells (presumably tumor cells and fibroblasts) and 30% were immune cells. One interesting observation is that DAL4-LNP could selectively _target mRNA to CD19+ B cells but not T cells (Fig. 3B). The data suggest that DAL4-LNP may display some unique epitopes that can selectively bind to B cells. DAL4-LNP may have the potential to be developed as therapeutics to address B cell malignancies.
We observed that local therapy using DAL4-LNP-IL-12 and DAL4-LNP-IL-27 mRNAs demonstrated a strong synergistic effect in inhibiting tumor growth. Our approach of localized cytokine delivery to the tumor displayed that IL-12 is the most potent anti-tumor agent amongst the three considered, which is consistent with previous studies that identified the role of IL-12 to promote Th1/Tc1 response7, 8 and to enhance T cell trafficking to tumors9. IL-27 is a member of the IL-12 family, and thus, promotes similar anti-tumor effects as IL-1246, 47. Despite similar capacities, IL-12 and IL-27 activate T cells and NK cells through different mechanisms: IL-12 activate T and NK cells via Stat4 pathway and IL-27 activates through Stat1 and Stat3 pathways. In addition to promoting Th1/Tc1 responses, we previously reported that IL-27 could enhance T cell survival in tumor microenvironment47, 48. These different properties of IL-27 and IL-12 explain the commensal effects of these two cytokines in both systemic therapy42 and localized therapy observed in this study. Another notable observation in this study is that the addition of GM-CSF does not further improve the antitumor effects of IL-12 and IL-27 mRNA LNPs. Although this observation requires further investigation, less effectiveness may be related to GM-CSF-mediated expansion of myeloid-derived suppressor cells49, 50, which could inhibit IL-12/IL-27-mediated anti-tumor immune responses and thereby promote tumor growth instead.
Our analysis indicates that intratumoral delivery of DAL4-LNP-IL-12 + IL-27 mRNA induced compelling anti-tumor immune responses. Notably, we observed a nearly 10-fold increase of total leukocyte infiltration within the TME after DAL4-LNP-IL-12 + IL-27 mRNA treatment. Furthermore, INF-γ and TNF-α producing T and NK cell effectors significantly increased in DAL4-LNP-IL-12 + IL-27 treated tumors, signaling an activation or stimulation of the recruited effector cells to secrete anti-tumor signaling molecules to further boost the anti-tumor response (Fig. 5). Sustained tumor inhibition after stopping treatment suggests that immune memory responses were established in treated mice. Currently, the lack of T lymphocyte infiltration51 has been considered to be a major factor responsible for low responding rates to anti-PD-1 therapy. Hence, it is tempting to hypothesize that our current therapeutic modality can potentially be used in combination with existing checkpoint inhibitors for enhanced efficacy.
A critical problem for cytokine-based cancer therapy is its narrow therapeutic window limited by severe adverse side effects3. Previously, the adoption of IL-12 therapy in clinics has been hindered by fatal toxicity5, 6. However, in this study, we establish that intratumoral injection of DAL4-LNP-IL-12 + IL-27 mRNA did not induce significant systemic toxicity in vivo, which is reflected by no significant body weight loss in treated mice or increase in overall fatality as compared to control-treated mice. Moreover, histology examination showed no inflammatory signatures were detected in major organs after DAL4-LNP-IL-12 + IL-27 mRNA administration. We speculate that two factors may play a role in mitigating systemic toxicity. First, the intratumoral injection approach may confine cytokine production to the TME with low systemic release. In the TME, the produced cytokines can be rapidly utilized by nearby immune cells. Indeed, previous studies have shown that intratumoral injection of LNPs can not only improve the local expression of the protein of interest but also reduce the systemic exposure of cargo16, 52, 53 Second, IL-27 is an anti-inflammatory cytokine that induces IL-10 production54–56. This property of IL-27 can potentially reduce the toxic inflammation caused by IL-12 alone. Therefore, dual IL-12+IL-27 mRNA DAL4-LNP delivery platform exhibits increased potency as well as decreased systemic toxicity.
Taken together, we disclose a new LNPs mediated mRNA delivery formulation and a cytokine combination that can induce robust tumor infiltration of immune effectors and inhibit tumor growth with reduced toxicity. The therapeutic modality can be further applied to expand current immunotherapy. Additionally, the delivery platform merits further development as a novel immunotherapeutic against cancer.
Supplementary Material
Acknowledgments
This work was supported by the Maximizing Investigators’ Research Award R35GM119679 from the National Institute of General Medical Sciences (YD) as well as by the National Cancer Institute grant R01CA229254 (XFB). C. X. Z. acknowledges the support from the Professor Sylvan G. Frank Graduate Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credited author statement
Jin-Qing Liu: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing - Review & Editing, Chengxiang Zhang: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing-Original Draft, Writing - Review & Editing, Xinfu Zhang: Validation, Investigation, Data Curation, Writing - Review & Editing, Jingyue Yan: Validation, Formal analysis, Investigation, Data Curation, Writing - Review & Editing, Chunxi Zeng: Validation, Investigation, Data Curation, Writing - Review & Editing, Fatemeh Talebian: Validation, Investigation, Data Curation, Writing - Review & Editing, Kimberly Lynch: Validation, Investigation, Data Curation, Writing - Review & Editing, Weiyu Zhao: Validation, Investigation, Data Curation, Writing - Review & Editing, Xucheng Hou: Validation, Investigation, Data Curation, Writing - Review & Editing, Shi Du: Validation, Investigation, Data Curation, Writing - Review & Editing, Diana D. Kang: Writing - Review & Editing, Binbin Deng: Validation, Investigation, Data Curation, Writing - Review & Editing, David W. McComb: Validation, Investigation, Data Curation, Writing - Review & Editing, Xue-Feng Bai: Conceptualization, Methodology, Validation, Formal analysis, Writing – Review & Editing, Visualization, Supervision, Project administration, Funding acquisition, Yizhou Dong: Conceptualization, Methodology, Validation, Formal analysis, Writing - Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Reference:
- [1].Borden EC: Interferons alpha and beta in cancer: therapeutic opportunities from new insights. Nat Rev Drug Discov 2019, 18:219–34. [DOI] [PubMed] [Google Scholar]
- [2].Mullard A: Restoring IL-2 to its cancer immunotherapy glory. Nat Rev Drug Discov 2021, 20:163–5. [DOI] [PubMed] [Google Scholar]
- [3].Waldmann TA: Cytokines in Cancer Immunotherapy. Cold Spring Harb Perspect Biol 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu JQ, Zhu J, Hu A, Zhang A, Yang C, Yu J, Ghoshal K, Basu S, Bai XF: Is AAV-delivered IL-27 a potential immunotherapeutic for cancer? Am J Cancer Res 2020, 10:3565–74. [PMC free article] [PubMed] [Google Scholar]
- [5].Car BD, Eng VM, Schnyder B, LeHir M, Shakhov AN, Woerly G, Huang S, Aguet M, Anderson TD, Ryffel B: Role of interferon-gamma in interleukin 12-induced pathology in mice. Am J Pathol 1995, 147:1693–707. [PMC free article] [PubMed] [Google Scholar]
- [6].Ryffel B: Interleukin-12: role of interferon-gamma in IL-12 adverse effects. Clin Immunol Immunopathol 1997, 83:18–20. [DOI] [PubMed] [Google Scholar]
- [7].Colombo MP, Trinchieri G: Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002, 13:155–68. [DOI] [PubMed] [Google Scholar]
- [8].Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A: Interleukin-12: biological properties and clinical application. Clin Cancer Res 2007, 13:4677–85. [DOI] [PubMed] [Google Scholar]
- [9].Hu J, Sun C, Bernatchez C, Xia X, Hwu P, Dotti G, Li S: T-cell Homing Therapy for Reducing Regulatory T Cells and Preserving Effector T-cell Function in Large Solid Tumors. Clin Cancer Res 2018, 24:2920–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhao Q, Hu J, Mitra A, Cutrera J, Zhang W, Zhang Z, Yan J, Xia X, Mahadeo KM, Livingston JA, Gorlick R, Li S: Tumor-_targeted IL-12 combined with tumor resection yields a survival-favorable immune profile. J Immunother Cancer 2019, 7:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L, Morgan RA, Gattinoni L, Rosenberg SA, Trinchieri G, Restifo NP: Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res 2010, 70:6725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang L, Morgan RA, Beane JD, Zheng Z, Dudley ME, Kassim SH, Nahvi AV, Ngo LT, Sherry RM, Phan GQ, Hughes MS, Kammula US, Feldman SA, Toomey MA, Kerkar SP, Restifo NP, Yang JC, Rosenberg SA: Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res 2015, 21:2278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mansurov A, Ishihara J, Hosseinchi P, Potin L, Marchell TM, Ishihara A, Williford JM, Alpar AT, Raczy MM, Gray LT, Swartz MA, Hubbell JA: Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat Biomed Eng 2020, 4:531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bhatia S, Longino NV, Miller NJ, Kulikauskas R, Iyer JG, Ibrani D, Blom A, Byrd DR, Parvathaneni U, Twitty CG, Campbell JS, Le MH, Gargosky S, Pierce RH, Heller R, Daud AI, Nghiem P: Intratumoral Delivery of Plasmid IL12 Via Electroporation Leads to Regression of Injected and Noninjected Tumors in Merkel Cell Carcinoma. Clin Cancer Res 2020, 26:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hewitt SL, Bailey D, Zielinski J, Apte A, Musenge F, Karp R, Burke S, Garcon F, Mishra A, Gurumurthy S, Watkins A, Arnold K, Moynihan J, Clancy-Thompson E, Mulgrew K, Adjei G, Deschler K, Potz D, Moody G, Leinster DA, Novick S, Sulikowski M, Bagnall C, Martin P, Lapointe JM, Si H, Morehouse C, Sedic M, Wilkinson RW, Herbst R, Frederick JP, Luheshi N: Intratumoral IL12 mRNA Therapy Promotes TH1 Transformation of the Tumor Microenvironment. Clin Cancer Res 2020, 26:6284–98. [DOI] [PubMed] [Google Scholar]
- [16].Li Y, Su Z, Zhao W, Zhang X, Momin N, Zhang C, Wittrup KD, Dong Y, Irvine DJ, Weiss R: Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nature Cancer 2020, 1:882–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Momin N, Mehta NK, Bennett NR, Ma L, Palmeri JR, Chinn MM, Lutz EA, Kang B, Irvine DJ, Spranger S, Wittrup KD: Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci Transl Med 2019, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Natividad KD, Junankar SR, Mohd Redzwan N, Nair R, Wirasinha RC, King C, Brink R, Swarbrick A, Batten M: Interleukin-27 Signaling Promotes Immunity against Endogenously Arising Murine Tumors. PLoS One 2013, 8:e57469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shinozaki Y, Wang S, Miyazaki Y, Miyazaki K, Yamada H, Yoshikai Y, Hara H, Yoshida H: Tumor-specific cytotoxic T cell generation and dendritic cell function are differentially regulated by interleukin 27 during development of anti-tumor immunity. Int J Cancer 2009, 124:1372–8. [DOI] [PubMed] [Google Scholar]
- [20].Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, Sun JC, Allison JP: Systemic 4–1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med 2013, 210:743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wei J, Xia S, Sun H, Zhang S, Wang J, Zhao H, Wu X, Chen X, Hao J, Zhou X, Zhu Z, Gao X, Gao JX, Wang P, Wu Z, Zhao L, Yin Z: Critical role of dendritic cell-derived IL-27 in antitumor immunity through regulating the recruitment and activation of NK and NKT cells. Journal of immunology 2013, 191:500–8. [DOI] [PubMed] [Google Scholar]
- [22].Liu Z, Liu JQ, Talebian F, Wu LC, Li S, Bai XF: IL-27 enhances the survival of tumor antigen-specific CD8(+) T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur J Immunol 2013, 43:468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T: Potent antitumor activity of interleukin-27. Cancer Res 2004, 64:1152–6. [DOI] [PubMed] [Google Scholar]
- [24].Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C, Shafer-Weaver K, Malyguine A, Kastelein R, Wigginton JM: IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. Journal of immunology 2004, 173:7170–82. [DOI] [PubMed] [Google Scholar]
- [25].Liu Z, Liu JQ, Shi Y, Zhu X, Liu Z, Li MS, Yu J, Wu LC, He Y, Zhang G, Bai XF: Epstein-Barr virus-induced gene 3-deficiency leads to impaired antitumor T-cell responses and accelerated tumor growth. Oncoimmunology 2015, 4:e989137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC: Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A 1993, 90:3539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Armstrong CA, Botella R, Galloway TH, Murray N, Kramp JM, Song IS, Ansel JC: Antitumor effects of granulocyte-macrophage colony-stimulating factor production by melanoma cells. Cancer Res 1996, 56:2191–8. [PubMed] [Google Scholar]
- [28].van Elsas A, Hurwitz AA, Allison JP: Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 1999, 190:355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dranoff G: GM-CSF-based cancer vaccines. Immunol Rev 2002, 188:147–54. [DOI] [PubMed] [Google Scholar]
- [30].Hege KM, Jooss K, Pardoll D: GM-CSF gene-modifed cancer cell immunotherapies: of mice and men. Int Rev Immunol 2006, 25:321–52. [DOI] [PubMed] [Google Scholar]
- [31].Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS: Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010, 363:411–22. [DOI] [PubMed] [Google Scholar]
- [32].Bommareddy PK, Patel A, Hossain S, Kaufman HL: Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am J Clin Dermatol 2017, 18:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, Leming P, Puzanov I, Shin D, Kirkwood JM: Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 2014, 312:1744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Luke JJ, Donahue H, Nishino M, Giobbie-Hurder A, Davis M, Bailey N, Ott PA, Hodi FS: Single Institution Experience of Ipilimumab 3 mg/kg with Sargramostim (GM-CSF) in Metastatic Melanoma. Cancer Immunol Res 2015, 3:986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tarhini AA, Joshi I, Garner F: Sargramostim and immune checkpoint inhibitors: combinatorial therapeutic studies in metastatic melanoma. Immunotherapy 2021, 13:1011–29. [DOI] [PubMed] [Google Scholar]
- [36].Hu A, Ding M, Zhu J, Liu JQ, Pan X, Ghoshal K, Bai XF: Intra-Tumoral Delivery of IL-27 Using Adeno-Associated Virus Stimulates Anti-tumor Immunity and Enhances the Efficacy of Immunotherapy. Front Cell Dev Biol 2020, 8:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang C, Zhang X, Zhao W, Zeng C, Li W, Li B, Luo X, Li J, Jiang J, Deng B, McComb DW, Dong Y: Chemotherapy drugs derived nanoparticles encapsulating mRNA encoding tumor suppressor proteins to treat triple-negative breast cancer. Nano Res 2019, 12:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang X, Zhao W, Nguyen GN, Zhang C, Zeng C, Yan J, Du S, Hou X, Li W, Jiang J, Deng B, McComb DW, Dorkin R, Shah A, Barrera L, Gregoire F, Singh M, Chen D, Sabatino DE, Dong Y: Functionalized lipid-like nanoparticles for in vivo mRNA delivery and base editing. Science Advances 2020, 6:eabc2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li B, Luo X, Deng B, Giancola JB, McComb DW, Schmittgen TD, Dong Y: Effects of local structural transformation of lipid-like compounds on delivery of messenger RNA. Scientific Reports 2016, 6:22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu JQ, Talebian F, Wu L, Liu Z, Li MS, Wu L, Zhu J, Markowitz J, Carson WE 3rd, Basu S, Bai XF: A Critical Role for CD200R Signaling in Limiting the Growth and Metastasis of CD200+ Melanoma. Journal of immunology 2016, 197:1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Talebian F, Yu J, Lynch K, Liu JQ, Carson WE, Bai XF: CD200 Blockade Modulates Tumor Immune Microenvironment but Fails to Show Efficacy in Inhibiting Tumor Growth in a Murine Model of Melanoma. Front Cell Dev Biol 2021, 9:739816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhu S, Lee DA, Li S: IL-12 and IL-27 sequential gene therapy via intramuscular electroporation delivery for eliminating distal aggressive tumors. Journal of immunology 2010, 184:2348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hou X, Zaks T, Langer R, Dong Y: Lipid nanoparticles for mRNA delivery. Nature Reviews Materials 2021, 6:1078–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li B, Luo X, Deng B, Wang J, McComb DW, Shi Y, Gaensler KML, Tan X, Dunn AL, Kerlin BA, Dong Y: An Orthogonal Array Optimization of Lipid-like Nanoparticles for mRNA Delivery in Vivo. Nano Letters 2015, 15:8099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cornebise M, Narayanan E, Xia Y, Acosta E, Ci L, Koch H, Milton J, Sabnis S, Salerno T, Benenato KE: Discovery of a Novel Amino Lipid That Improves Lipid Nanoparticle Performance through Specific Interactions with mRNA. Advanced Functional Materials, n/a:2106727. [Google Scholar]
- [46].Yoshimoto T, Chiba Y, Furusawa J, Xu M, Tsunoda R, Higuchi K, Mizoguchi I: Potential clinical application of interleukin-27 as an antitumor agent. Cancer Sci 2015, 106:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhu J, Liu JQ, Shi M, Cheng X, Ding M, Zhang JC, Davis JP, Varikuti S, Satoskar AR, Lu L, Pan X, Zheng P, Liu Y, Bai XF: IL-27 gene therapy induces depletion of Tregs and enhances the efficacy of cancer immunotherapy. JCI Insight 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu Z, Liu JQ, Talebian F, Wu LC, Li S, Bai XF: IL-27 enhances the survival of tumor antigen-specific CD8+ T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur J Immunol 2013, 43:468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Morales JK, Kmieciak M, Knutson KL, Bear HD, Manjili MH: GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1-bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res Treat 2010, 123:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rosborough BR, Castellaneta A, Natarajan S, Thomson AW, Turnquist HR: Histone deacetylase inhibition facilitates GM-CSF-mediated expansion of myeloid-derived suppressor cells in vitro and in vivo. J Leukoc Biol 2012, 91:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A: PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Li W, Zhang X, Zhang C, Yan J, Hou X, Du S, Zeng C, Zhao W, Deng B, McComb DW, Zhang Y, Kang DD, Li J, Carson WE, Dong Y: Biomimetic nanoparticles deliver mRNAs encoding costimulatory receptors and enhance T cell mediated cancer immunotherapy. Nature Communications 2021, 12:7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hewitt SL, Bailey D, Zielinski J, Apte A, Musenge F, Karp R, Burke S, Garcon F, Mishra A, Gurumurthy S, Watkins A, Arnold K, Moynihan J, Clancy-Thompson E, Mulgrew K, Adjei G, Deschler K, Potz D, Moody G, Leinster DA, Novick S, Sulikowski M, Bagnall C, Martin P, Lapointe J-M, Si H, Morehouse C, Sedic M, Wilkinson RW, Herbst R, Frederick JP, Luheshi N: Intratumoral IL12 mRNA Therapy Promotes TH1 Transformation of the Tumor Microenvironment. Clinical Cancer Research 2020, 26:6284–98. [DOI] [PubMed] [Google Scholar]
- [54].Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA: Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 2007, 8:1363–71. [DOI] [PubMed] [Google Scholar]
- [55].Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL: A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 2007, 8:1380–9. [DOI] [PubMed] [Google Scholar]
- [56].Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A: Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. Journal of immunology 2007, 179:3268–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


