Abstract
Strong biofilm-forming Enterococcus feacalis urinary tract pathogens (n = 35) were used to determine the lytic spectrum of six bacteriophages isolated from sewage samples. Only 17 Enterococcus feacalis isolates gave lytic zones with the tested bacteriophages from which five isolates were susceptible to all of them. The isolated enterococcal phages are characterized by wide range of thermal (30–90 °C) and pH (3–10) stability. They belong to order Caudovirales, from which four bacteriophages (EPA, EPB, EPD, EPF) belong to family Myoviridae and two (EPC, EPE) belong to family Siphoviridae. In addition, they have promising antibiofilm activity against the tested strong-forming biofilm E. faecalis isolates. The enterococcal phages reduced the formed and preformed biofilms to a range of 38.02–45.7% and 71.0–80.0%, respectively, as compared to the control. The same promising activities were obtained on studying the anti-adherent effect of the tested bacteriophages on the adherence of bacterial cells to the surface of urinary catheter segments. They reduced the number of adherent cells to a range of 30.8–43.8% and eradicated the pre-adherent cells to a range of 48.2–71.1%, as compared to the control. Overall, the obtained promising antibiofilm activity makes these phages good candidates for application in preventing and treating biofilm associated Enterococcus faecalis infections.
Subject terms: Drug discovery, Microbiology
Introduction
Enterococcus species are commensals gram-positive bacteria which cause many infections in susceptible hosts, such as endocarditis, bacteremia, oral infections and urinary tract infections (UTI) especially device associated infections. In Egyptian UTI patients, Enterococcus faecalis (E. faecalis) is found to be the second highest prevalence after Escherichia coli. UTIs in hospitalized, catheterized and diabetic patients are higher than outpatients1,2. One of the virulence factors of Enterococcus besides the enzymes and proteins secreted is its ability to form biofilm3.
The National Institutes of Health (NIH) revealed that among all microbial and chronic infections, 65% and 80%, respectively, are associated with biofilm formation. These include both, device and non-device-associated infections4. Catheters associated urinary tract infections (CAUTI) account for 40% of all nosocomial infections, of which 15% to 30% are associated with E. faecalis and Enterococcus faecium (E. faecium)5,6.
E. faecalis lives as commensal organism in the gastrointestinal tract. Nonetheless, it may trigger life-threatening infections including endocarditis, bacteremia, UTI and meningitis, and it is particularly common in hospitals where antibiotic resistance has grown7. The devices associated enterococcal infections, like CAUTI, are mainly due to biofilm associated bacteria8.
Biofilm forming bacteria show resistance to many antibiotics and immune response which results in treatment failure9. Given the difficulty of treating and eradicating biofilm associated infections, there is an unmet need for therapeutic options other than antibiotics to prevent biofilm formation. New approaches were developed to overcome this problem, among which was bacteriophage therapy10.
Bacteriophages are the natural predators for bacteria, and are the most common biological entities on the planet; phage particles are usually five to ten times more abundant than bacterial cells. So, phage predation is thought to cut the global bacterial population in half every 48 h11. Bacteriophages have many advantages over antibiotics therapy; they can affect all types of bacteria including multi drug resistant ones, they are promising in the economic aspect, and finally, being natural products, they are likely to be devoid of apparent toxicity12. These characteristics make bacteriophages an attractive non-antibiotic therapy for treating biofilm associated bacterial infections13.
The aim of the present study was to investigate the effect of different isolated bacteriophages on the planktonic and biofilm associated E. faecalis urinary tract pathogens. To achieve this, bacteriophages were isolated from sewage, followed by their characterization, and in vitro assessment of their lytic and antibiofilm activity against biofilm associated E. faecalis urinary tract pathogens.
Results
Identification of bacterial isolates and assessment of biofilm formation
All the collected clinical isolates (n = 100) can grow on Bile Esculin agar. In addition, the isolates are gram positive and catalase positive confirming that all the isolates are Enterococcus species.
The results of evaluating the biofilm formation showed that 35 isolates formed strong biofilm (OD range 0.24–2.66) (Supplementary Table S1), while the other isolates were either moderate (n = 29) or weak/non-biofilm forming (n = 36). The identification of the strong biofilm producer isolates (n = 35) was further confirmed by VITEK and the results showed that all the isolates were Enterococcus faecalis.
Isolation and purification of bacteriophages from sewage samples
Six phages were isolated from the community samples, of which four bacteriophages (EPA, EPB, EPC, EPD) were isolated from Kalyoub governorate’s samples and two (EPE, EPF) from Zagazig governorate’s samples. On the other hand, no bacteriophage could be isolated from hospitals and clinics’ samples. The isolated bacteriophages were identified by formation of clear plaques on bacterial lawns. Four phages showed hallow zone around the plaques and the other two showed plaques with defined boundaries.
Characterization of bacteriophages
Determination of host range
When the isolated phages were spotted on the surface of plates seeded with the strong biofilm forming isolates (n = 35), only 17 bacterial isolates gave clear plaques from which five bacterial isolates were susceptible to all the tested bacteriophages (Table 1).
Table 1.
Lytic activity spectra of 6 different enterococcal phages (EPA: F) against different enterococcal isolates (n = 17).
| Isolates codes | EPA | EPB | EPC | EPD | EPE | EPF |
|---|---|---|---|---|---|---|
| EF11 | – | – | √ | √ | √ | √ |
| EF16 | √ | √ | √ | √ | – | – |
| EF20 | – | – | – | √ | – | – |
| EF38 | √ | √ | √ | √ | √ | √ |
| EF43 | √ | √ | √ | √ | √ | √ |
| EF48 | √ | √ | – | – | – | – |
| EF80 | – | – | √ | – | √ | – |
| EF99 | – | – | √ | √ | √ | – |
| EF101 | √ | √ | √ | √ | √ | √ |
| EF103 | √ | √ | – | √ | √ | – |
| EF113 | √ | – | √ | √ | √ | – |
| EF114 | – | √ | √ | √ | √ | √ |
| EF125 | √ | √ | – | √ | √ | √ |
| EF133 | √ | √ | √ | √ | √ | √ |
| EF134 | √ | √ | √ | √ | √ | √ |
| EF146 | √ | – | – | – | – | – |
| EF151 | √ | – | √ | √ | – | – |
Isolate codes (EF)*: are the codes for E. faecalis isolates.
(√) indicates bacterial lysis, (–) indicates absence of plaques (no lysis).
Plaque morphology
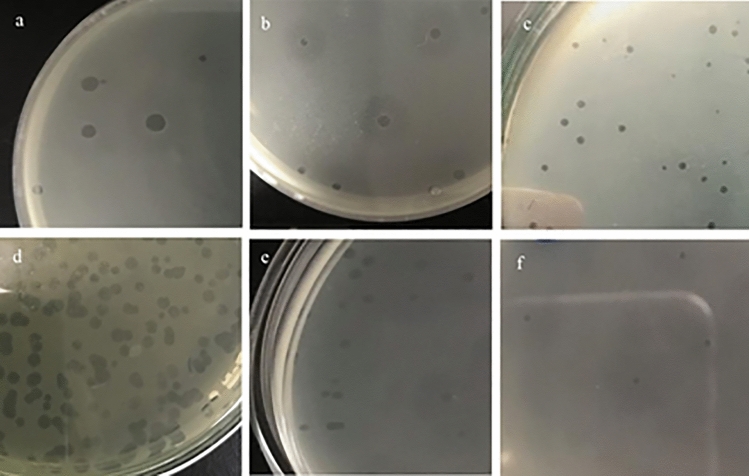
The formed plaques are circular. Four bacteriophages (EPA, EPE, EPC, EPD) showed plaques with diameters of range 1–4 mm. while, the other two bacteriophages (EPB, EPF) produced plaques with diameters less than 1 mm (Fig. 1). Hallow zones around the plaques were produced by only four bacteriophages (EPA, EPB, EPE, EPF).
Figure 1.
Plaques pictures of the 6 bacteriophages (EPA: F), as formed on Enterococcus double layer agar plates. (a) EPA: large plaques (diameter: up to 4 mm) with hallow zones. (b) EPB: medium plaques (diameter: 2–3 mm) with hallow zone. (c) EPC: medium plaques (diameter: 1–2 mm) without hallow zones. (d) EPD: large plaques (diameter: up to 4 mm) without hallow zone. (e) EPE: medium plaques (diameter: 1 mm) with hallow zone. (f) EPF: pinpoint plaques (diameter: < 1 mm) with hallow zone.
Effect of temperature on bacteriophages’ stability
All the bacteriophages showed lytic activity against the five enterococcal isolates (EF38, EF43, EF101, EF133 and EF134), are sensitive to all the tested bacteriophages, after exposure to temperature 40, 50, and 70 °C for 1 h. On exposure to 90 °C, the three phages EPD, EPE and EPF caused promising lytic activity to all the tested isolates. In addition, only EPD phage induced lytic activity after exposure to 100 °C (Supplementary Table S2).
Effect of pH on bacteriophages’ stability
The experiment was carried against the five enterococcal isolates that are sensitive to all the tested bacteriophages; EF 38, EF 43, EF 101, EF 133 and EF 134. The obtained results revealed that the six bacteriophages did not lose their infectivity after 1 h exposure to all the tested pH values (3–11).
Transmission electron microscope visualization
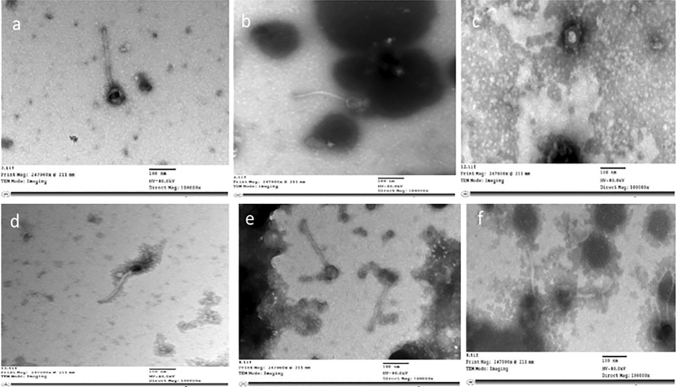
According to the data of transmission electron microscope and the international committee on taxonomy of viruses, the 6 bacteriophages are tailed phages belong to order Caudovirales, from which 4 bacteriophages (EPA, EPB, EPD, EPF) belong to family Myoviridae and had heads (dimension ranges: 53.8–119 × 59.5–108 nm) attach to long and contractile tails (159–197 nm length and 9.03–13.6 nm width). In addition, the other two bacteriophages (EPC, EPE) belong to family Siphoviridae possess heads (dimension ranges: 48.6–63.8 × 53.1–62.8 nm) to which long, flexible and non-contractile tails (204–212 nm length and 5.97–9.9 nm width) were attached14,15 (Table 2, Fig. 2A–F).
Table 2.
Dimensions of the six enterococcal phages (EPA: F) by using transmission electron microscope.
| Bacteriophage | Head (nm) | Tail length × width (nm) |
|---|---|---|
| EPA | 119 × 108 | 159 × 9.03 |
| EPB | 63.8 × 65.9 | 194 × 13.6 |
| EPC | 63.8 × 62.8 | 212 × 9.9 |
| EPD | 55.3 × 61.4 | 197 × 10.4 |
| EPE | 48.6 × 53.1 | 204 × 5.97 |
| EPF | 53.8 × 59.5 | 189 × 9.73 |
Figure 2.
TEM images of the six different enterococcal bacteriophages (a) phage EPA, (b) phage EPB, (c) phage EPC, (d) phage EPD, (e) phage EPE, (f) phage EPF, with scale bar 100 nm.
Antibiofilm effect of bacteriophages
Prevention of biofilm formation
Different bacterial dilutions (1/10, 1/100, 1/500, 1/1000) of the selected Enterococcus isolates (n = 17) were used against the six tested bacteriophages with titer 107. Results were calculated as an average for every specific dilution and then as a collective average for the six bacteriophages. The results of antibiofilm of the tested bacteriophages showed that the average of percentages of the formed biofilms as compared to the control on using the prepared bacterial dilution were 49.3, 41.4, 52.6 and 49.5% respectively. The lowest percentage of biofilm formation was obtained by using 1/500 dilution (Fig. 3).
Figure 3.
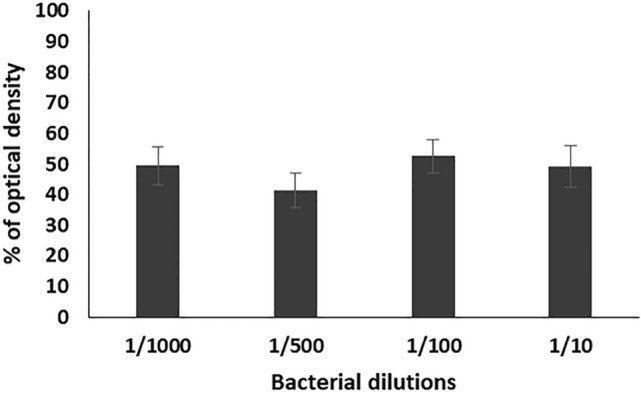
Percentage of optical density of the formed biofilms of 17 enterococcal isolates, prepared in different dilutions (1/10, 1/100, 1/500, and 1/1000) from bacterial suspension adjusted to 0.5 MacFarland, in the presence of the six bacteriophages (EPA, EPB, EPC, EPD, EPE, EPF) as compared to the control. Each bar represents the mean of three independent measurements.
The results of antibiofilm effect of the tested bacteriophages (EPA, EPB, EPC, EPD, EPE, EPF) against the tested enterococcal isolates at dilution 1/500 showed reduction in the formed biofilms to 39.9, 45.7, 38, 42.1, 39.2, 44% as compared to that of the control, respectively (Fig. 4).
Figure 4.
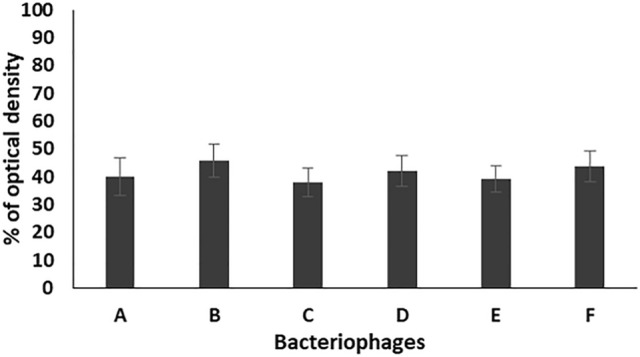
Percentage of optical density of the formed biofilms of 17 enterococcal isolates, prepared in 1/500 dilution from bacterial suspension adjusted to 0.5 MacFarland, in the presence of the six bacteriophages (A: EPA, B: EPB, C: EPC, D: EPD, E: EPE, F: EPF) as compared to the control. Each bar represents the mean of three independent measurements.
Bacteriophage activity on preformed biofilm
Results were calculated as an average for every specific bacterial dilution and then as a collective average for the six bacteriophages. The obtained results showed that the tested bacteriophages caused reduction in the preformed biofilms by different bacterial dilutions 1/10, 1/100, 1/500, and 1/1000, to the averages of 85.1, 82.3, 76 and 88% as compared to the control, respectively. The lowest percentage of biofilm formation was obtained at dilution 1/500 (Fig. 5).
Figure 5.
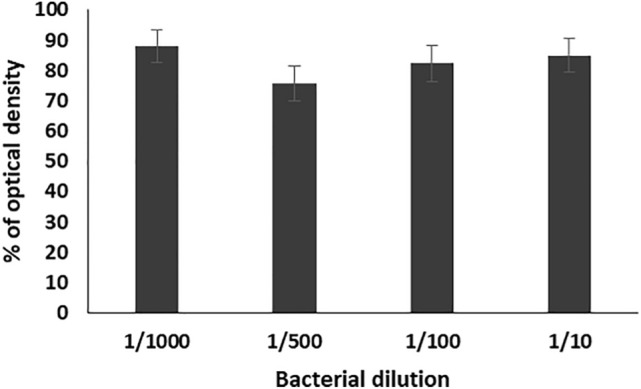
Percentage of the formed biofilms of 17 enterococcal isolates, prepared in different dilutions (1/10, 1/100, 1/500, and 1/1000) from bacterial suspension adjusted to 0.5 MacFarland, after treatment the preformed biofilms with the six bacteriophages (EPA, EPB, EPC, EPD, EPE, EPF) as compared to the control. Each bar represents the mean of three independent measurements.
This bacterial dilution (1/500) was used to evaluate the efficiency of the tested bacteriophages in eradicating the preformed biofilms by 17 enterococcal isolates. The obtained results revealed that the bacteriophages EPA, EPB, EPC, EPD, EPE, EPF caused reduction in the OD of the preformed biofilm to 78.4, 76, 73.3, 75, 71, 80% as compared to the control, respectively (Fig. 6).
Figure 6.
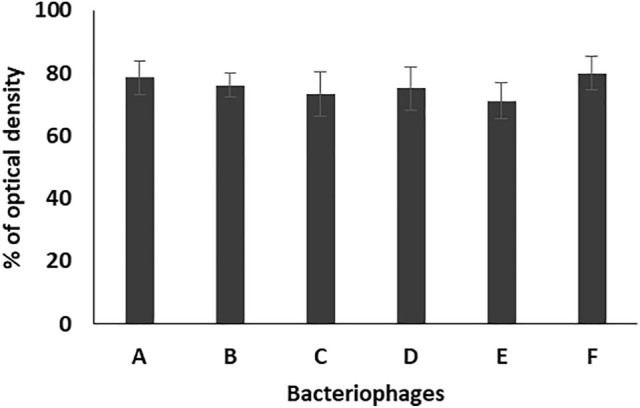
Percentage of the formed biofilms of 17 enterococcal isolates, prepared in different dilutions (1/10, 1/100, 1/500, and 1/1000) from bacterial suspension adjusted to 0.5 MacFarland, after treatment the preformed biofilms with the six bacteriophages (A: EPA, B: EPB, C: EPC, D: EPD, E: EPE, F:EPF) as compared to the control. Each bar represents the mean of three independent measurements.
Effect of bacteriophage on bacterial adherence to urinary catheter surfaces
The three strongest biofilm producer enterococcal isolates (EF104, EF134, and EF151) and the phages (EPA, EPC and EPE) that showed the highest antibiofilm activity were used in this experiment.
The obtained results showed that the presence of the bacteriophages EPA, EPC and EPE reduced the number of adherent enterococcal cells to 30.8, and 35.3, 43.8%, respectively, as compared to that of the control (Fig. 7).
Figure 7.
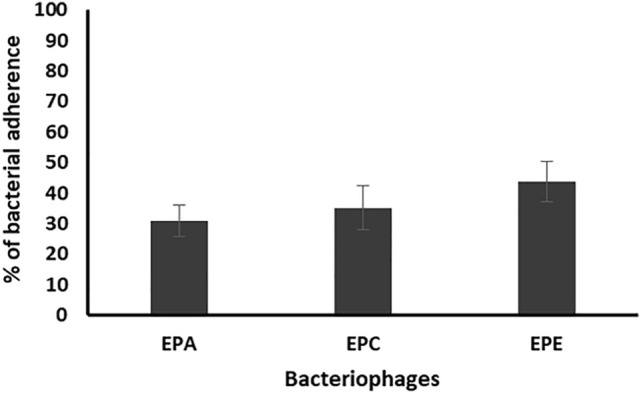
Percentage of bacterial adherence on the surface of catheters segments of 3 enterococcal isolates (EF104, EF134, and EF151), prepared in dilution 1/500 from bacterial suspension adjusted to 0.5 MacFarland, in the presence of the three bacteriophages (EPA, EPC, EPE) as compared to the control. Each bar represents the mean of three independent measurements.
In addition, treatment of the enterococcal preformed biofilm on urinary catheter surfaces with the selected bacteriophages caused reduction in the number of adherent cells to 48.2, 54.2, 71.1%, respectively, as compared to that of the control (Fig. 8).
Figure 8.
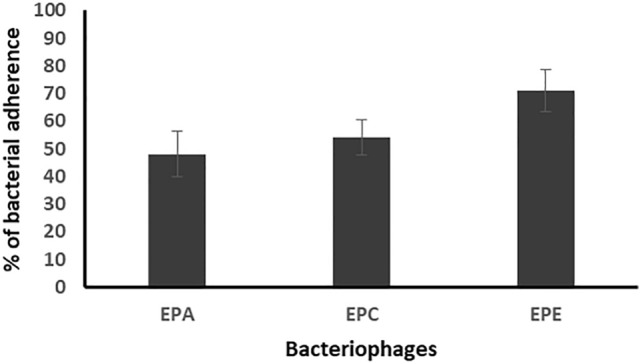
Percentage of bacterial adherence on the surface of catheters segments of the 3 enterococcal isolates (EF104, EF134, and EF151), prepared in dilution 1/500 from bacterial suspension adjusted to 0.5 MacFarland, after treatment the preformed biofilms with the three bacteriophages (EPA, EPC, EPE) as compared to the control. Each bar represents the mean of three independent measurements.
Discussion
Urinary tract infections (UTIs) are one of the most frequent human bacterial infections. Enterococcal UTIs are of particularly concerned as they are radically resistant to first-line antimicrobial agents, especially vancomycin16. Also, Enterococcus strains have an extraordinary potential to form biofilms that are difficult to eradicate17. This fact together with the concern that humankind is reentering the “pre-antibiotics” era, have caused the development of alternative anti-infective practices to be one of the highest considerations of modern medicine.
Being one of the promising therapeutic approaches, phage therapy has several advantages that have made it potentially attractive over antibiotics due to phages’ high specificity, low toxicity to humans, self-amplification and liability to rapid modification to fight newly arising bacterial threats18,19. The specificity of phage action on host cell depends on the structure of the receptor on the bacterial surface that involved in phage adsorption which initiates the phage infection to the host bacterial cell and followed by process of phage replication and host cell lysis20.
In the present study, out of one hundred enterococcal urinary tract clinical isolates, 35% (n = 35) were found to form strong biofilm which increases the importance of finding a treatment for them. This result is close to that reported by Zhi-jian Yu et al. who isolated 113 samples from UTI patients and found 26.5% of them were strong biofilm forming21.
On screening for a specific enterococcal phage therapy, six bacteriophages were isolated from sewage water. Based on previous studies22, bacteriophages were successfully isolated from samples collected at governorate’s sewage site in Cairo, Egypt. This was attributed to the sewage rich contents of bacteria and organic matters23. On the other hand, no bacteriophage could be isolated from hospitals and clinics’ wastewater samples. This may be due to the excessive use of disinfectants in the hospitals and clinics that finally released into the wastewater, especially during the period of corona virus pandemic.
The increase in enteroccocal phage titers in the bacterial culture confirms the phage infection and replication in host cells, ending with bacterial cell lysis that is confirmed by plaque formation. The size of the formed plaques was varied (< 1–0.1–4 mm in diameter) after 24 h of incubation at 37 °C. In addition, hallow zones around the plaques were produced by only four bacteriophages (EPA, EPB, EPE, EPF) and the other two (EPC and EPD) showed plaques with defined boundaries. The isolated enterococcal bacteriophages showed lytic activity against seventeen E. faecalis isolates, five of which were sensitive to all the tested bacteriophages but the remaining isolates showed different sensitivity patterns. The lytic effect of tested bacteriophages on biofilm associated Enterococcus isolates gave an indication for their ability to penetrate the biofilm reaching to the host bacterial cells, in agreement with the previous reports that showing the efficacy of bacteriophage to reduce bacterial biofilm24.
Upon characterization using TEM, four of the isolated phages (EPA, EPB, EPD, EPF) having long and contractile tails, were found to be related to the family Myoviridae and the other two bacteriophages (EPC and EPE), having long, flexible and non-contractile tails, are related to family Siphoviridae. This finding gave another support that such phages are effective against the Enterococcus species, since most of the viruses related to both Myovirdae and Siphoviridae families are potential predators of enterobacteria, according to the international committee on taxonomy of viruses25. In addition previous studies identified different bacteriophages related to family Myoviridae26,27 and Siphoviridae22,28 showed promising antibiofilm activity against different types of biofilm associated bacteria.
Regarding the thermal and pH stability, all the tested E. faecalis phages showed excellent stability at temperature between 40 and 70 °C for 1 h. Whereas, three bacteriophages (EPD, EPE, EPF) survived at 90 °C and only one bacteriophage (EPD) retained its lytic activity at 100 °C. Moreover, all the bacteriophages showed stability at pH between 3 and 11. The stability of the tested phages at wide range of temperature and pH gave them advantage for preparation in suitable pharmaceutical form and used for therapeutic purpose. In addition, the stability of the phage at acidic and alkaline conditions (pH 3–11) facilitates the product to be administered through oral route without any change in the viability along the gastrointestinal tract.
Our findings nearly conceded those reported by a previous work that evaluated stability of Staphylococcus bacteriophages at different temperature and pH values29. However, E. faecalis phage PHB08 isolated by Yang et al. showed stability at temperature and pH ranges of 4–60 °C and 5.0–9.0, respectively30. Also, ValSw3-3 bacteriophage displayed a stability at temperature between 4 and 50 °C and pH between 3 and 10, respectively31. In addition, Goodarzi et al. investigated that the isolated enterococcal phage S2 showed stability when exposed to a wide range of pH values (1 to 11)22.
For studying the antibiofilm activity of the enterococcal phages, four different bacterial dilutions (1/1000, 1/500, 1/100, 1/10) were used. The results revealed that all the tested phages can prevent the biofilm formation and eradicate the preformed ones of the respective Enterococcus isolates. The averages of the formed biofilms were 49.5%, 41.4%, 52.6%, and 49.3% for bacterial dilutions 1/1000, 1/500, 1/100, and 1/10, respectively, as compared to that of the control. Also, treatment of preformed biofilm by the same dilutions caused reduction in the biofilm to 88.1, 75.6, 82.3, 85.1%, respectively, as compared to the control. Accordingly, the best antibiofilm activities induced by tested bacteriophages were obtained on using bacterial dilution 1/500 at which the percentage of the remained biofilm was significantly lower than that of all the other dilutions (p-value < 0.05). In addition, the three enterococcal bacteriophages (EPA, EPC and EPE) had the strongest antibiofilm activity. Therefore, bacterial dilution 1/500 was used for evaluating the efficacy of the three bacteriophages EPA, EPC and EPE to prevent adherence of three of strong biofilm producer enterococcal isolates (EF104, EF134, and EF151) to the surface of urinary catheter segments. The obtained results confirmed the ability of the selected bacteriophages to prevent biofilm formation and adherence of the tested isolates onto the surface of the urinary catheter through reducing the number of the adherent cells to an average of 30.8, and 35.3, 43.8%, respectively, as compared to that of the control. In addition, they efficiently eradicated the pre-adherent cells on the catheter surface and reduced their number to the average of 48.2, 54.2, 71.13%, respectively, as compared to the control.
The ability of the tested enterococcal tailed phages to invade the biofilm matrix and cause lysis to the bacterial cells in the deeper layer results from the hydrolytic activity of depolymerase enzymes that present in their tail spike proteins32,33 or the presence of endolysins that have remarkable activity to lyse planktonic cells34,35. Accordingly, the findings of this study support the notion that bacteriophages can be used as a promising alternative strategy to conventional antibiotic therapy to prevent and treat the UTIs and also urinary catheter associated infections caused by biofilm producing E. faecalis pathogens.
Materials and methods
Collection of Enterococcus isolates and culture conditions
One hundred enterococcal clinical isolates were obtained from different Microbiology Laboratories in Cairo (Al-Borg laboratories) and Alexandria (Al Shatby Hospital). The obtained isolates were taken after the laboratories finished the analysis process on these isolates. According to the obtained information, all the isolates were Enterococcus species and isolated from urine samples of urinary tract infected patients. Purification and identification of the collected isolates were done by surface streaking on Bile Esculin agar (oxoid), microscopical examination, and catalase test36. VITEK system (Biomerieux, USA), was carried out at El Zahraa University teaching hospital to identify the species of some of the obtained enterococcal isolates.
The isolates were stored at −80 °C in 25% glycerol in nutrient broth. Before use, the isolates were plated from glycerol stocks onto Luria Bertani (LB) agar and incubated at 37 °C for 24 h.
Assessment of biofilm formation by microtiter plate assay
For preparation of fresh bacterial suspension, one colony from the isolate grown on LB agar plates was inoculated in 5 mL of tryptone soy broth with 1% glucose (TSBG) and incubated at 37 °C for 24 h. The obtained cultures were first adjusted to 0.5 MacFarland (1 × 108 CFU/mL) using spectrophotometer V-630 (Unicam, UK) and then diluted 1:100 with fresh medium (106 CFU/mL). Individual wells of sterile 96 wells flat bottom tissue culture plate were filled with 200 µL of the diluted cultures and incubated at 37 °C for 24 h. After incubation, contents of each well were removed by gentle tapping, then washed with 0.2 mL of phosphate buffer saline (PBS, pH 7.2) three times to remove planktonic cells. Biofilms formed by adherent bacteria were fixed by methanol and stained by 0.1% crystal violet solution (Oxford, UK). Excess stain was removed by using deionized water and plates were kept for drying. The dyes adhered to bacterial cells were resolubilized by 33% glacial acetic acid (Fisher Chemical, US). Optical density (OD) of stained adherent biofilm was obtained by using micro-ELISA auto-reader (ELx-800, BiotTek, UK) at wavelength 630 nm. Wells containing bacteria free medium were used as controls37. Measurements were performed in triplicate and the average was calculated. As described by Mathur et al., the strength of the biofilm formation was classified into strong (OD > 0.240), moderate (OD = 0.120–0.240), and weak and non-biofilm producer (OD < 0.120)38.
Isolation and purification of bacteriophages from sewage samples
Twelve sewage samples were collected from different locations in Egypt; hospitals (Al Shatby, Alexandria and El Kasr El ainy, Cairo, Egypt), clinics and community (Kalyoub and Zagazig cities, Egypt). Sewage samples (4.5 mL) were mixed with 0.5 mL of overnight culture of Enterococcus sp. in tryptone soy broth (TSB) and 5 mL fresh double strength TSB in falcon tube and incubated in incubator shaker at 37 °C and 150 rpm. Enriched samples were then centrifuged (Sigma 2-16 KL refrigerated centrifuge, Germany) at 6700×g for 20 min at 4 °C. Filtration of the supernatant was done using a 0.45 μm pore-size disposable syringe filter (Cellulose acetate, sterile) to remove any remaining bacterial cells. Screening for presence of phages by plaques demonstration was done using the double layer agar (DLA) method as described by Frederick39. In brief, 100 µL of the bacteriophage and 200 µL of overnight bacterial samples were added to 3 mL of sterile tryptone soy soft agar (TSA) and mix gently then poured on 10 mL hard TSA in petri dish. After solidification, the plates were incubated in inverted position at 37 °C for 24 h to visualize the developed plaques.
To obtain a purified bacteriophage, sterile tip was gently stabbed in the focal point of a single plaque and then put into 1 mL of TSB and pipetted to deliver bacteriophage particles into broth. The filtrate was ten times diluted and used to proceed in DLA method to observe the formed plaques. The same steps of isolation were repeated for purification and enrichment. This method was rehashed until pure plaques appeared40.
Characterization of bacteriophages
Determination of host range of infection
An aliquot (10 µL) of phage sample was spot inoculated onto Tryptone soy DLA seeded with one of the strong biofilm producers enterococcal isolates. The plates were then incubated inverted for 24 h at 37 °C and the plaques formation examined on the next day41.
Plaque morphology
Plaques morphology was characterized through determination of diameter size, clearance and presence of halo zone around the plaques42.
Effect of temperature on bacteriophages’ stability
Thermal stability of bacteriophages was measured by exposure of the enterococcal phages to five different temperatures: 40, 50, 70, 90 and 100 °C for 1 h. Then spot test method was carried out for checking the survival of bacteriophages29.
Effect of pH on bacteriophages’ stability
Enterococcal bacteriophages were exposed to different pH values ranging from 3 to 11 using 0.1 M NaCl and 0.1 M HCL, at temperature of 37 °C for 1 h. Then, spot test method was carried out for checking the survival of bacteriophages29.
Transmission electron microscope (TEM) visualization
An aliquot (5 µL) of phage lysate (1010 PFU/mL) was transferred to carbon grid and air dried for 5 min, then 5 µL of negative stain phosphotungstic acid was added and air dried for 10 min. Images of the phages were captured, at Cairo university research park, using transmission electron microscope (JEM-1400 EM, Japanese Electron Optic Ltd.) which was set at 60 kV and magnification power of 10,000×42.
Antibiofilm effect of bacteriophages
Prevention of biofilm formation
Fresh culture of strong biofilm producer E. faecalis isolate was used to prepare bacterial suspension in TSBG broth and adjusted to 0.5 MacFarland as described before and then diluted to 1/10, 1/100, 1/500 and 1/1000 with fresh medium. Individual wells of sterile 96 well flat bottom tissue culture plates were filled with 100 µL of the diluted cultures and 50 µL of bacteriophages of titer 107, then the plates were incubated at 37 °C for 24 h. After incubation, the wells’ liquid contents were aspirated and the process of washing, staining and measuring the OD of the adherent biofilm was carried out as described above. Wells contained TSB inoculated with tested bacterial isolate were used as control24.
Treatment of preformed biofilm
As described before, 200 µL of diluted bacterial culture in TSBG (105 CFU/mL) was used to fill each well in 96 well flat bottom plates and incubated at 37 °C for 24 h. After incubation, the liquid media was aspirated and the wells were washed thrice using PBS to remove any planktonic cells, followed by adding 200 µL bacteriophage lysate with titer 107 and incubated for 24 h. The phage lysate was removed after incubation and the wells were washed, stained, and OD of the remaining biofilm was determined. Wells contained only TSB inoculated only with tested bacterial isolate were used as control24.
Effect of bacteriophage on bacterial adherence to urinary catheter surfaces
Prevention of bacterial adherence to catheter surfaces
Three pieces (each 1 cm length) of urinary catheter segments (size 14) were placed in Wassermann tubes containing 0.5 mL of phage lysate with titer 107 for 1 h at 37 °C, then 1 mL of bacterial suspension adjusted to 0.5 McFarland and diluted 1:500 with TSBG was added and continue incubation for 24 h. After incubation, each catheter segment was removed and washed thrice with PBS to remove planktonic cells and then placed in new Wassermann tube with 1 mL saline. For dislodgment of the adherent cell from catheter surface, the segment in saline was sonicated for 30 s three times at 20 kHz, then counting of the released cells was carried out by drop plate method. For each sample, counting of the viable bacterial cells was carried out in triplicates and the mean value was calculated. The segments that were placed in bacterial suspension without previous treatment with phage lysate were used as control43,44.
Eradication of preformed bacterial biofilm on catheter surfaces
Three pieces of urinary catheter segments were incubated with 1 mL bacterial suspension for 24 h at 37 °C to form bacterial biofilm on catheter surfaces. After incubation, the segments were removed and washed with PBS three times to remove planktonic cells and each segment was transferred to new tube containing 1 mL TSBG inoculated with phage lysate with titer 107 and incubated for 24 h at 37 °C. After incubation, the number of adherent bacterial cells to segment surface was determined as described before. The segments that were placed in bacterial suspension without treatment with phage lysate were used as control45,46.
Statistical analysis
The obtained data were converted to percentage as compared to that of the control. Then statistical analysis (one-way ANOVA test) was carried out by using GraphPad Prism (GraphPad Soſtware Tools, Inc., La Jolla, CA, USA) to evaluate the effect of the isolated bacteriophages on the biofilm associated E. faecalis urinary tract pathogens. Data were presented as mean ± standard deviation and a p-value less than 0.05 was considered statistically significant.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplementary Information
Author contributions
M.A.Y., R.F.E. and A.M.A. conceived and designed the study, D.M.E. performed the practical experiments and acquisition of data, M.A.Y., R.F.E., A.M.A. and D.M.E. analyzed and interpreted the data; M.A.Y., R.F.E., A.M.A. and D.M.E. contributed in the writing and revising the manuscript, all authors read and approved the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17275-z.
References
- 1.Turjeman A, et al. Risk factors for enterococcal urinary tract infections: A multinational, retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:2005–2010. doi: 10.1007/s10096-021-04207-4. [DOI] [PubMed] [Google Scholar]
- 2.Abdelkareem MZ, Sayed M, Hassuna NA, Mahmoud MS, Abdelwahab SF. Multi-drug-resistant Enterococcus faecalis among Egyptian patients with urinary tract infection. J. Chemother. 2017;29:74–82. doi: 10.1080/1120009X.2016.1182358. [DOI] [PubMed] [Google Scholar]
- 3.Stępień-Pyśniak D, Hauschild T, Kosikowska U, Dec M, Urban-Chmiel R. Biofilm formation capacity and presence of virulence factors among commensal Enterococcus spp. from wild birds. Sci. Rep. 2019;9:1–7. doi: 10.1038/s41598-019-47602-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamal M, et al. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018;81:7–11. doi: 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Oumer Y, Dadi BR, Seid M, Biresaw G, Manilal A. Catheter-associated urinary tract infection: Incidence, associated factors and drug resistance patterns of bacterial isolates in southern ethiopia. Infect. Drug Resist. 2021;14:2883. doi: 10.2147/IDR.S311229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guiton PS, Hung CS, Hancock LE, Caparon MG, Hultgren SJ. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect. Immun. 2010;78:4166–4175. doi: 10.1128/IAI.00711-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebreton, F., Willems, R. J. & Gilmore, M. S. Enterococcus diversity, origins in nature, and gut colonization. in Enterococci: From Commensals to Leading Causes of Drug Resistant Infection (2014). [PubMed]
- 8.Swidan NS, Hashem YA, Elkhatib WF, Yassien MA. Antibiofilm activity of green synthesized silver nanoparticles against biofilm associated enterococcal urinary pathogens. Sci. Rep. 2022;12:1–13. doi: 10.1038/s41598-022-07831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dostert M, Trimble MJ, Hancock RE. Antibiofilm peptides: Overcoming biofilm-related treatment failure. RSC Adv. 2021;11:2718–2728. doi: 10.1039/D0RA09739J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira VC, et al. Bacteriophage cocktail-mediated inhibition of Pseudomonas aeruginosa biofilm on endotracheal tube surface. Antibiotics. 2021;10:78. doi: 10.3390/antibiotics10010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrix RW. Bacteriophages: Evolution of the majority. Theor. Popul. Biol. 2002;61:471–480. doi: 10.1006/tpbi.2002.1590. [DOI] [PubMed] [Google Scholar]
- 12.Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5:226–235. doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatfull GF, Dedrick RM, Schooley RT. Phage therapy for antibiotic-resistant bacterial infections. Annu. Rev. Med. 2022;73:197–211. doi: 10.1146/annurev-med-080219-122208. [DOI] [PubMed] [Google Scholar]
- 14.Fokine A, Rossmann MG. Molecular architecture of tailed double-stranded DNA phages. Bacteriophage. 2014;4:e28281. doi: 10.4161/bact.28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackermann H-W. Tailed bacteriophages: The order Caudovirales. Adv. Virus Res. 1998;51:135–201. doi: 10.1016/S0065-3527(08)60785-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Solache M, Rice LB. The Enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 2019;32:e00058–e118. doi: 10.1128/CMR.00058-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg S, Mohan B, Taneja N. Biofilm formation capability of enterococcal strains causing urinary tract infection vis-a-vis colonisation and correlation with enterococcal surface protein gene. Indian J. Med. Microbiol. 2017;35:48–52. doi: 10.4103/ijmm.IJMM_16_102. [DOI] [PubMed] [Google Scholar]
- 18.Woitschach F, et al. Bacterial adhesion and biofilm formation of Enterococcus faecalis on zwitterionic methylmethacrylat and polysulfones. Front. Cell. Infect. Microbiol. 2022;1:557. doi: 10.3389/fcimb.2022.868338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adesanya O, Oduselu T, Akin-Ajani O, Adewumi OM, Ademowo OG. An exegesis of bacteriophage therapy: An emerging player in the fight against anti-microbial resistance. AIMS Microbiol. 2020;6:204. doi: 10.3934/microbiol.2020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doub JB, Urish K, Lee M, Fackler J. Impact of bacterial phenotypic variation with bacteriophage therapy: A pilot study with prosthetic joint infection isolates. Int. J. Infect. Dis. 2022;119:44–46. doi: 10.1016/j.ijid.2022.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Zheng J-X, et al. Characterization of biofilm formation by Enterococcus faecalis isolates derived from urinary tract infections in China. J. Med. Microbiol. 2018;67:60. doi: 10.1099/jmm.0.000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodarzi F, et al. Biological characteristics and anti-biofilm activity of a lytic phage against vancomycin-resistant Enterococcus faecium. Iran. J. Microbiol. 2021;13:691. doi: 10.18502/ijm.v13i5.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SN, Armbruster CE. Indwelling urinary catheter model of Proteus mirabilis infection. Methods Mol. Biol. 2019;2019:187–200. doi: 10.1007/978-1-4939-9601-8_17. [DOI] [PubMed] [Google Scholar]
- 24.Adnan M, et al. Isolation and characterization of bacteriophage to control multidrug-resistant Pseudomonas aeruginosa planktonic cells and biofilm. Biologicals. 2020;63:89–96. doi: 10.1016/j.biologicals.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Lefkowitz EJ, et al. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV) Nucleic Acids Res. 2018;46:D708–D717. doi: 10.1093/nar/gkx932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan FM, et al. A novel Acinetobacter baumannii bacteriophage endolysin LysAB54 with high antibacterial activity against multiple Gram-negative microbes. Front. Cell. Infect. Microbiol. 2021;11:637313. doi: 10.3389/fcimb.2021.637313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yüksel FN, Buzrul S, Akçelik M, Akçelik N. Inhibition and eradication of Salmonella Typhimurium biofilm using P22 bacteriophage, EDTA and nisin. Biofouling. 2018;34:1046–1054. doi: 10.1080/08927014.2018.1538412. [DOI] [PubMed] [Google Scholar]
- 28.Wintachai P, Voravuthikunchai SP. Characterization of novel lytic Myoviridae phage infecting multidrug-resistant Acinetobacter baumannii and synergistic antimicrobial efficacy between phage and Sacha Inchi oil. Pharmaceutical. 2022;15:291. doi: 10.3390/ph15030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmoud, E. R. A., Ahmed, H. A. H., Abo-senna, A. S. M., Riad, O. K. M. & Abo, M. M. A. A. R. Isolation and characterization of six gamma-irradiated bacteriophages specific for MRSA and VRSA isolated from skin infections. J. Radiat. Res. Appl. Sci.14, 34–43 (2021).
- 30.Yang D, et al. Characterization of a lytic bacteriophage vB_EfaS_PHB08 harboring endolysin Lys08 against Enterococcus faecalis biofilms. Microorganisms. 2020;8:1332. doi: 10.3390/microorganisms8091332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, et al. Characterization and genomic analysis of ValSw3-3, a new Siphoviridae bacteriophage infecting Vibrio alginolyticus. J. Virol. 2020;94:e00066–e120. doi: 10.1128/JVI.00066-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dakheel KH, et al. Proteomic analysis revealed the biofilm-degradation abilities of the bacteriophage UPMK_1 and UPMK_2 against Methicillin-resistant Staphylococcus aureus. Biotechnol. Lett. 2022;44:513–522. doi: 10.1007/s10529-022-03229-y. [DOI] [PubMed] [Google Scholar]
- 33.Abedon ST. Ecology of anti-biofilm agents I: antibiotics versus bacteriophages. Pharmaceuticals. 2015;8:525–558. doi: 10.3390/ph8030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh A, Padmesh S, Dwivedi M, Kostova I. How good are bacteriophages as an alternative therapy to mitigate biofilms of nosocomial infections. Infect. Drug Resist. 2022;15:503. doi: 10.2147/IDR.S348700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutierrez D, Ruas-Madiedo P, Martínez B, Rodríguez A, García P. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS ONE. 2014;9:e107307. doi: 10.1371/journal.pone.0107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray BE. The life and times of the Enterococcus. Clin. Microbiol. Rev. 1990;3:46–65. doi: 10.1128/CMR.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen GD, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathur T, et al. Detection of biofilm formation among the clinical isolates of staphylococci: An evaluation of three different screening methods. Indian J. Med. Microbiol. 2006;24:25–29. doi: 10.1016/S0255-0857(21)02466-X. [DOI] [PubMed] [Google Scholar]
- 39.Santos SB, et al. The use of antibiotics to improve phage detection and enumeration by the double-layer agar technique. BMC Microbiol. 2009;9:1–10. doi: 10.1186/1471-2180-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukman C, Yonathan C, Magdalena S, Waturangi DE. Isolation and characterization of pathogenic Escherichia coli bacteriophages from chicken and beef offal. BMC Res. Notes. 2020;13:1–7. doi: 10.1186/s13104-019-4859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan Mirzaei M, Nilsson AS. Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE. 2015;10:e0118557. doi: 10.1371/journal.pone.0118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jurczak-Kurek A, et al. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016;6:1–17. doi: 10.1038/srep34338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LewisOscar F, et al. In vitro analysis of green fabricated silver nanoparticles (AgNPs) against Pseudomonas aeruginosa PA14 biofilm formation, their application on urinary catheter. Prog. Org. Coat. 2021;151:106058. doi: 10.1016/j.porgcoat.2020.106058. [DOI] [Google Scholar]
- 44.Rakov C, et al. _targeting biofilm of MDR Providencia stuartii by phages using a catheter model. Antibiotics. 2021;10:375. doi: 10.3390/antibiotics10040375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Townsend EM, Moat J, Jameson E. CAUTI’s next top model–Model dependent Klebsiella biofilm inhibition by bacteriophages and antimicrobials. Biofilm. 2020;2:100038. doi: 10.1016/j.bioflm.2020.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu W, et al. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob. Agents Chemother. 2010;54:397–404. doi: 10.1128/AAC.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.