Abstract
Gradual depletion of oxygen causes the shift-down of aerobic growing Mycobacterium bovis BCG to an anaerobic synchronized state of nonreplicating persistence. The persistent culture shows induction of glycine dehydrogenase and α-crystallin-like protein and is sensitive to metronidazole.
Mycobacterium tuberculosis is capable of causing asymptomatic latent infection. In the latent infection, bacilli persist for years before they reactivate and cause active tuberculosis (5, 14, 21). How the tubercle bacillus survives during the latent stage of infection is largely unknown. Following initial infection, the bacilli typically replicate inside host macrophages until an effective immune response is mounted and the bacilli become restricted to the characteristic tuberculous lesions and the progression of the disease is halted (4). The bacillus can survive in the caseous necrotic center of these lesions, but it apparently cannot multiply because of oxygen deprivation and other adverse conditions (4). It is within this anaerobic environment of the caseous necrotic material that bacterial dormancy probably occurs.
Lawrence Wayne showed in vitro that oxygen depletion indeed triggers a dormancy response of the bacillus (21, 22). In the Wayne model, cultures of the bacterium are subjected to gradual self-generated oxygen depletion by incubation in sealed stirred tubes. Upon the slow shift of aerobic growing M. tuberculosis to anaerobic conditions, the culture is able to adapt and survive anaerobiosis by shifting down to a state of nonreplicating persistence (9, 22). Three growth phases can be observed. After initial aerobic exponential growth, the turbidity of the culture increases slowly without a corresponding increase in viable counts. This phase was termed NRP-1 (nonreplicating persistence stage 1). After NRP-1, the culture enters a phase where no further increase in turbidity is seen. This phase was named NRP-2. A key feature of the dormancy response is that the anaerobic persistent NRP-2 culture is arrested at a uniform stage of the cell cycle, i.e., the culture is synchronized (18, 22). Glycine dehydrogenase activity of the bacillus was shown to be induced during NRP-1. During NRP-2, this enzyme activity declines somewhat but stays above the aerobic baseline level (19, 22). Recently, the 16-kDa α-crystallin-like small heat shock protein was shown to be induced upon oxygen limitation in vitro (3, 23), and its upregulation correlates with a thickening of the cell wall (3). Intriguingly, an induction of this protein was also demonstrated in macrophages (24). Furthermore, anaerobic persistent bacilli develop sensitivity to metronidazole (20, 22), a drug specific for anaerobes (8). However, first analyses of the action of metronidazole on persisting organisms in experimental murine tuberculosis are inconclusive due to contradictory results (6, 13).
The vaccine strain Bacille Calmette-Guèrin (BCG) is an attenuated form of Mycobacterium bovis and thus closely related to the tubercle bacillus. Although BCG is widely used as a live vaccine (1 billion children have been vaccinated since 1921 [10]), little is known about its long-term persistence in the human host. However, reports of AIDS patients developing BCG adenitis and disseminated infection 30 years after being vaccinated indicate that BCG might be able to persist in the body (1, 15). The experimental disadvantages associated with the pathogenic nature of M. tuberculosis prompted us to investigate the possibility of using BCG as a nonpathogenic model for dormancy. Thus, we asked whether the oxygen depletion-induced dormancy response shown by M. tuberculosis in the Wayne culture model is conserved in BCG Pasteur ATCC 35734.
Anaerobic survival and synchronized reactivation of BCG.
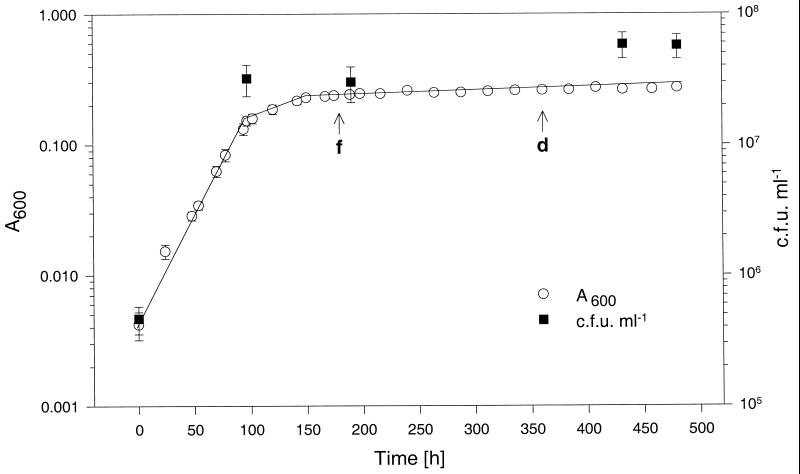
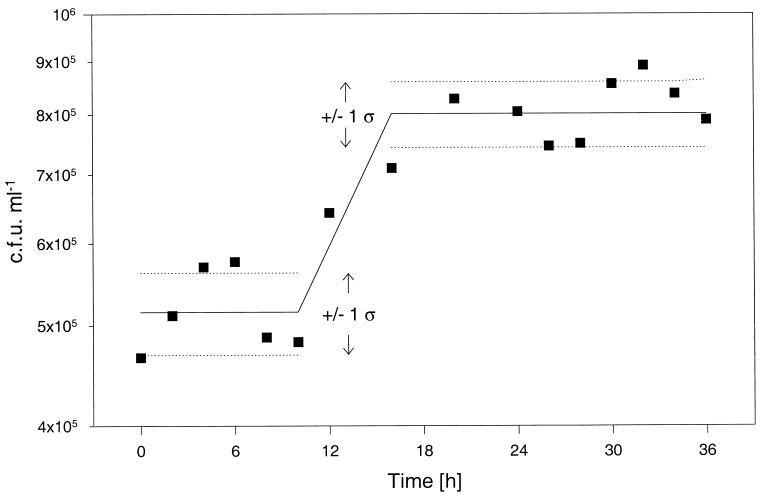
Figure 1 shows the growth and survival of BCG in the Wayne dormancy model, i.e., under sealed stirred culture conditions. After an initial aerobic growth phase (19-h generation time) the culture entered an NRP-1 phase which was characterized by a slow increase of turbidity without an increase in viable counts. The culture then entered an NRP-2 phase in which the turbidity stayed constant. As is the case for M. tuberculosis, a slow depletion of oxygen was observed (Fig. 1). Fading of the oxygen indicator methylene blue did not start until after termination of NRP-1. Complete decolorization took about an extra 8 days. These results show that growth, adaptation, and survival of BCG under the Wayne dormancy culture conditions are essentially identical to those of the tubercle bacillus. To determine whether the persistent NRP-2 culture of BCG was synchronized, reactivation experiments were carried out. Anaerobic sealed cultures (at 20 days) were diluted 1:100 in fresh oxygen-rich medium and incubated with aeration. Figure 2 shows synchronous cell division upon reactivation after a lag phase of about 13 h.
FIG. 1.
Growth and survival of BCG under the conditions of the Wayne dormancy culture model. Log A600 as a function of time is shown. Viable counts at selected time points are indicated. An aerobic exponential preculture was diluted to A600 = 0.005 (5 × 105 CFU ml−1) with Dubos Tween-albumin broth (Difco) and incubated in sealed tubes under gentle stirring conditions, as described previously (7, 22). f and d indicate fading and complete decolorization of the oxygen indicator methylene blue. Mean values and standard deviations from five independent experiments are shown. Viable counts were determined by plating appropriate dilutions of the cultures on Dubos oleic albumin agar. For all CFU determinations in this report, cultures were checked microscopically for any clumping of cells. Significant clumping was never observed.
FIG. 2.
Synchronous cell division after reactivation of anaerobic persistent BCG. Log CFUs per milliliter as a function of time after reactivation of an anaerobic persistent culture are shown. Twenty-day-old sealed stirred culture was diluted 1:100 in fresh oxygen-rich medium and incubated under aerated conditions. The data points represent mean values from three independent experiments. The solid lines between 0 and 10 h and between 16 and 36 h represent the average of the respective mean counts (n = 6 and 9) during those periods of shift-up. The dotted lines represent 1 standard deviation above and below these averages.
Induction of glycine dehydrogenase.
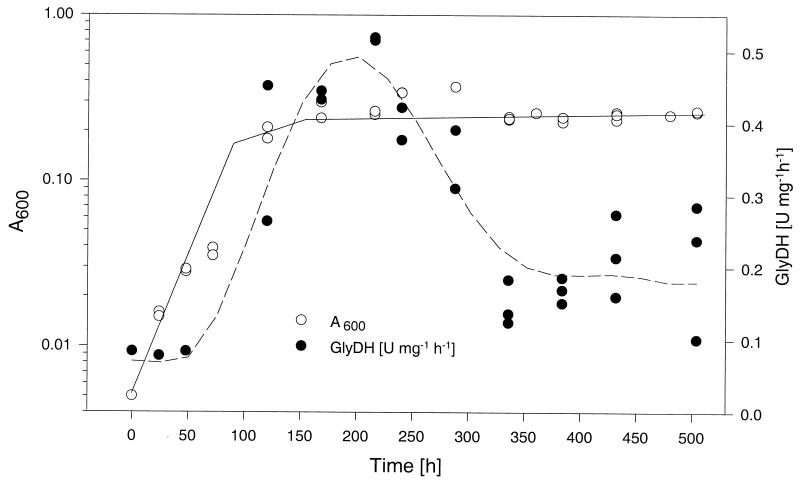
To analyze the temporal profile of glycine dehydrogenase activity during growth under sealed stirred culture conditions, protein extracts were prepared at various time points by using a Bead Beater (Biospec). Glycine dehydrogenase activity was determined by an assay based on optical measurement of the rate of oxidation of NADH to NAD, a reaction that accompanies the reductive amination of glyoxylate to glycine (19). Protein concentrations were determined with a Bio-Rad kit. Figure 3 shows that the specific enzyme activity stayed at a low baseline level during the initial aerobic exponential growth phase (0.08 U mg−1 h−1). After termination of aerobic growth, glycine dehydrogenase activity increased rapidly during NRP-1 to a level sixfold greater than the baseline level. During NRP-2, the enzyme activity declined until it reached a level about 40% of that of the peak value. These results show that the temporal profile of glycine dehydrogenase activity in BCG grown under the Wayne culture conditions is strikingly similar to the profile observed for M. tuberculosis.
FIG. 3.
Induction of glycine dehydrogenase activity during growth of BCG under the conditions of the Wayne dormancy culture model. The temporal profile of specific glycine dehydrogenase (GlyDH) activity in sealed stirred cultures is shown. An aerobic exponential preculture was diluted A600 = 0.005 and incubated in sealed tubes under gentle stirring conditions. Protein extracts were prepared at various time points, and specific glycine dehydrogenase activities were determined by using a photometric assay. The corresponding growth curve monitored by A600 determination is shown. This figure is based on data from two independent experiments.
An increase of isocitrate lyase activity, a marker of the glyoxylate shunt, has been reported for oxygen-deprived cultures of sedimented tubercle bacilli (19). However, determination of isocitrate lyase activity (16) in aerated growing (79 ± 12 μmol mg−1 min−1) and anaerobic persistent 20-day-old BCG cultures (7 ± 1 μmol mg−1 min−1) showed that this enzyme activity is down regulated in anoxic BCG grown under the Wayne culture conditions. Isocitrate lyase activity of anoxic tubercle bacilli grown under the Wayne culture conditions has not been reported yet.
Induction of α-crystallin-like protein.
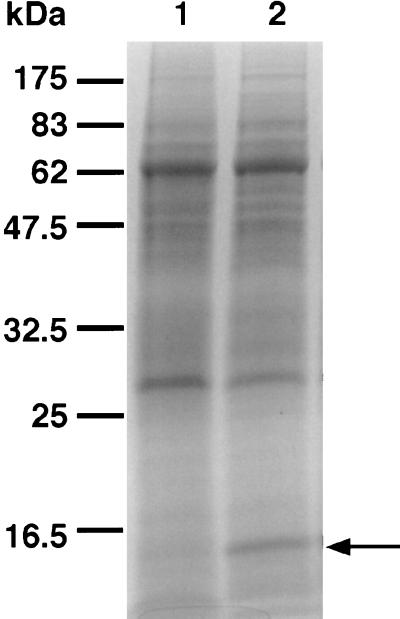
To assess whether the 16-kDa α-crystallin-like protein was induced in BCG under the conditions of the Wayne dormancy model, protein extracts from aerated exponentially growing and anaerobic persistent 20-day-old cultures were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Figure 4 shows that a high level of a 16-kDa protein was detectable in the extract from anaerobic persistent cultures but not in the extract from aerated growing cultures. To confirm the identity of the 16-kDa protein, the Coomassie-stained band was excised from the gel and digested with trypsin, and one of the tryptic peptides was sequenced on an Applied Biosystems Procise Sequencer. With the resulting amino acid sequence DGQLTIKA, a search of the M. tuberculosis genome database (Sanger Centre [2]) was carried out. The only match with 100% sequence identity was the α-crystallin-like protein. The induction of α-crystallin-like protein in BCG grown under the Wayne dormancy culture conditions is consistent with recent findings showing induction of this protein in BCG under oxygen-limiting conditions (3, 17, 24).
FIG. 4.
Induction of α-crystallin-like protein in anaerobic persistent BCG culture. A Coomassie-blue-stained 12% sodium dodecyl sulfate polyacrylamide gel of total protein extracts is shown. Lanes: 1, extract from aerated, vigorously shaking, exponentially growing BCG culture (A600 = 0.2); 2, extract from anaerobic persistent 20-day-old culture containing the upregulated 16-kDa α-crystallin-like protein (arrow). The molecular masses of the protein standards are indicated.
Development of metronidazole sensitivity.
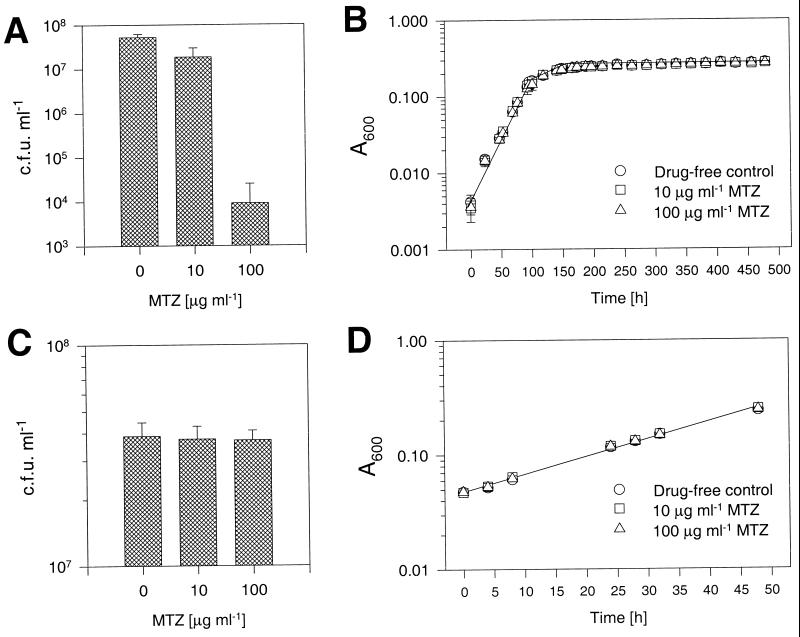
To determine whether anaerobic dormant BCG develops sensitivity to metronidazole, cultures were incubated in the presence of various concentrations of the drug under sealed stirred conditions. Metronidazole was added at the beginning of the experiment, and survival of the bacilli was determined after 20 days of incubation. Metronidazole was found to have a dose-dependent bactericidal effect on anaerobic BCG. Figure 5A shows that 10 and 100 μg ml−1 metronidazole reduced viable counts threefold and more than 1,000-fold, respectively. Aerated, exponentially growing cultures were not affected by the drug (Fig. 5C).
FIG. 5.
Development of metronidazole sensitivity in BCG grown under the conditions of the Wayne dormancy culture model. (A and B) Aerated exponentially growing precultures were diluted to A600 = 0.005 (5 × 105 CFU ml−1). Metronidazole (0, 10, and 100 μg ml−1) was added, and the cultures were sealed and gently stirred for 20 days. (A) Survival of bacilli after 20 days of incubation is plotted as a function of metronidazole (MTZ) concentration. MTZ is observed to have a bactericidal effect. (B) Monitoring of A600 as a function of time shows that the loss of viability was not accompanied by a decline in the turbidity of the culture. (C and D) Metronidazole resistance of BCG grown under aerated conditions. Aerated exponentially growing precultures were diluted to A600 = 0.05 (5 × 106 CFU ml−1). Metronidazole (0, 10, and 100 μg ml−1) was added, and the unsealed cultures were vigorously shaken. In order to stay within the exponential growth phase, the cultures were terminated after 2 days of incubation. (C) Survival of bacilli after 2 days of incubation is plotted as a function of metronidazole (MTZ) concentration. MTZ is observed to have no effect on the aerobically growing cultures. (D) Monitoring of A600 as a function of time did not reveal any differences in the generation time of metronidazole-free and metronidazole-containing cultures. Mean values and standard deviations from three independent experiments are shown.
Conclusions.
We recently described a dormancy response, induced by oxygen depletion, for Mycobacterium smegmatis (7). This physiological response, first observed in M. tuberculosis (21), appears to be conserved between the slow-growing pathogen and the fast-growing saprophyte. There are, however, several molecular differences between the dormancy response in M. smegmatis and that of the tubercle bacillus (11, 12, 23). In this paper, we report that BCG is capable of adapting to anaerobiosis by shifting down to a persistent state in a manner similar to the tubercle bacillus. While the vaccine strain is slow growing and therefore less convenient for molecular genetic analyses, we demonstrate that its physiological behavior is strikingly similar to that of the closely related pathogenic M. tuberculosis. Furthermore, the expression of two molecular markers, α-crystallin-like protein and glycine dehydrogenase, in BCG is very similar to that in M. tuberculosis. It has also recently been demonstrated that microaerobically and anaerobically cultured BCG developed a thickened cell wall similar to that of M. tuberculosis (3). These morphological, physiological, and molecular observations suggest BCG may be a useful nonpathogenic model for the in vitro analyses of dormancy of the tubercle bacillus.
Acknowledgments
We thank IMCB’s Protein Micro Sequencing Laboratory for peptide sequencing. We thank L. G. Wayne for discussion.
This study was supported by the Institute of Molecular and Cell Biology (IMCB).
REFERENCES
- 1.Armbruster C, Junker W, Vetter N, Jaksch G. Disseminated bacille Calmette-Guerin infection in an AIDS patient 30 years after BCG vaccination. J Infect Dis. 1990;162:1216. doi: 10.1093/infdis/162.5.1216. [DOI] [PubMed] [Google Scholar]
- 2.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M A, Rogers J, Rutter S, Seeger K, Skelton J, Squares S, Squares R, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham A F, Spreadbury C L. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J Bacteriol. 1998;180:801–808. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dannenberg A M, Jr, Rook G A W. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses. In: Bloom B R, editor. Tuberculosis. Washington, D.C: ASM Press; 1994. pp. 459–483. [Google Scholar]
- 5.DeMaio J, Zhang Y, Ko C, Young D B, Bishai W R. A stationary-phase stress-response sigma factor from Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1996;93:2790–2794. doi: 10.1073/pnas.93.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhillon J, Allen B W, Hu Y M, Coates A R M, Mitchison D A. Metronidazole has no antibacterial effect in Cornell model murine tuberculosis. Int J Tuberc Lung Dis. 1998;2:736–742. [PubMed] [Google Scholar]
- 7.Dick T, Lee B H, Murugasu-Oei B. Oxygen depletion induced dormancy in Mycobacterium smegmatis. FEMS Microbiol Lett. 1998;163:159–164. doi: 10.1111/j.1574-6968.1998.tb13040.x. [DOI] [PubMed] [Google Scholar]
- 8.Edwards D I. Nitroimidazole drugs—action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y M, Butcher P D, Sole K, Mitchison D A, Coates A R M. Protein synthesis is shut down in dormant Mycobacterium tuberculosis. FEMS Microbiol Lett. 1998;158:139–145. doi: 10.1111/j.1574-6968.1998.tb12813.x. [DOI] [PubMed] [Google Scholar]
- 10.Huebner R E. BCG vaccination in the control of tuberculosis. In: Shinnick T M, editor. Tuberculosis. Berlin, Germany: Springer-Verlag; 1996. pp. 263–282. [DOI] [PubMed] [Google Scholar]
- 11.Hutter B, Dick T. Increased alanine dehydrogenase activity during dormancy in Mycobacterium smegmatis. FEMS Microbiol Lett. 1998;167:7–11. doi: 10.1111/j.1574-6968.1998.tb13200.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee B H, Murugasu-Oei B, Dick T. Upregulation of a histone-like protein in dormant Mycobacterium smegmatis. Mol Gen Genet. 1998;260:475–479. doi: 10.1007/s004380050919. [DOI] [PubMed] [Google Scholar]
- 13.Paramasivan C N, Kubendiran G, Herbert D. Action of metronidazole in combination with isoniazid and rifampicin on persisting organisms in experimental murine tuberculosis. Indian J Med Res. 1998;108:115–119. [PubMed] [Google Scholar]
- 14.Parrish N M, Dick J D, Bishai W R. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6:107–112. doi: 10.1016/s0966-842x(98)01216-5. [DOI] [PubMed] [Google Scholar]
- 15.Reynes J, Perez C, Lamaury I, Janbon F, Bertrand A. Bacille Calmette-Guerin adenitis 30 years after immunization in a patient with AIDS. J Infect Dis. 1989;160:727. doi: 10.1093/infdis/160.4.727. [DOI] [PubMed] [Google Scholar]
- 16.Rua J, Soler J, Busto F, de Arriaga D. The pH dependence and modification by diethyl pyrocarbonate of isocitrate lyase from Phycomyces blakesleeanus. Eur J Biochem. 1995;232:381–390. doi: 10.1111/j.1432-1033.1995.tb20822.x. [DOI] [PubMed] [Google Scholar]
- 17.Tabira Y, Ohara N, Ohara N, Kitaura H, Matsumoto S, Naito M, Yamada T. The 16-kDa α crystallin-like protein of Mycobacterium bovis BCG is produced under conditions of oxygen deficiency and is associated with ribosomes. Res Microbiol. 1998;149:255–264. doi: 10.1016/s0923-2508(98)80301-x. [DOI] [PubMed] [Google Scholar]
- 18.Wayne L G. Synchronized replication of Mycobacterium tuberculosis. Infect Immun. 1977;17:528–530. doi: 10.1128/iai.17.3.528-530.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wayne L G, Lin K Y. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 1982;37:1042–1049. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wayne L G, Sramek H A. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1994;38:2054–2058. doi: 10.1128/aac.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wayne L G. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis. 1994;13:908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 22.Wayne L G, Hayes L G. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan Y, Crane D D, Barry C E., III Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J Bacteriol. 1996;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan Y, Crane D D, Simpson R M, Zhu Y, Hickey M J, Sherman D R, Barry C E., III The 16-kDa α-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc Natl Acad Sci USA. 1998;95:9578–9583. doi: 10.1073/pnas.95.16.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]