Abstract
BACKGROUND
After acute myocardial infarction (AMI), treatment with beta-blockers and angiotensin-converting enzyme inhibitors (ACEI) is widely recognized as crucial to reduce risk of a subsequent AMI. However, many patients fail to consistently remain on these treatments over time, and long-term adherence has not been well described.
OBJECTIVE
To examine the duration of treatment with beta-blockers and ACEI within the 24 months after an AMI.
DESIGN
A retrospective, observational study using medical and pharmacy claims from a large health plan operating in the Northeastern United States.
SUBJECTS
Enrollees with an inpatient claim for AMI who initiated beta-blocker (N = 499) or ACEI (N = 526) therapy.
MEASUREMENT
Time from initiation to discontinuation was measured with pharmacy refill records. Associations between therapy discontinuation and potential predictors were estimated using a Cox proportional hazards model.
RESULTS
ACEI discontinuation rates were high: 7% stopped within 1 month, 22% at 6 months, 32% at 1 year and 50% at 2 years. Overall discontinuation rates for beta-blockers were similar, but predictors of discontinuation differed for the two treatment types. For beta-blockers, the risk of discontinuation was highest among males and those from low-income neighborhoods; patients with comorbid hypertension and peripheral vascular disease were less likely to discontinue therapy. These factors were not associated with ACEI discontinuation.
CONCLUSION
Many patients initiating evidence-based secondary prevention therapies after an AMI fail to consistently remain on these treatments. Adherence is a priority area for development of better-quality measures and quality-improvement interventions. Barriers to beta-blocker adherence for low-income populations need particular attention.
KEY WORDS: acute myocardial infarction, adherence, beta-blockers, angiotensin converting enzyme inhibitors (ACEI), secondary prevention
INTRODUCTION
Approximately 1.2 million acute myocardial infarctions (AMI) occur each year in the United States, resulting in 180,000 deaths.1 The risk of reinfarction is significant, and prevention is a major public health challenge.1 There is extensive evidence supporting the use of beta-adrenergic blocking agents (beta-blockers) and angiotensin-converting enzyme inhibitors (ACEI) for secondary prevention of AMI.2–4 Guidelines for the management of AMI from the American College of Cardiology and the American Heart Association (ACC/AHA) recommend beta-blocker and ACEI therapy for all patients without contraindications, and use of angiotensin II receptor blockers (ARB), for patients who are intolerant of ACEI or show signs of heart failure.4 In light of the evidence and guideline recommendations, prescription rates for AMI patients upon hospital discharge have appropriately received attention in the last several years. There have been many quality improvement efforts in hospitals to increase the rates of discharge prescriptions for beta-blockers and ACEI. Prescription rates at discharge are considered core performance measures for tracking quality of care provided to AMI patients.5 Improvements in these rates have been observed through the 1990s and early 2000s, with current rates nationally reaching 93% at hospital discharge for members of commercial managed care plans.5,6
Improving rates of appropriate prescriptions at discharge is an important step, but another aspect of post-AMI care that is of critical importance and has received less attention is the extent to which prescribed therapies are maintained over time. The ACC/AHA guidelines recommend that these therapies be used indefinitely.4 Limited data suggest that many patients who initiated these regimes after AMI have low rates of long-term persistence.7–10 Beta-blocker or ACEI therapies are indicated for other diseases (e.g., coronary artery disease and hypertension), and studies following patients with such conditions also demonstrated low long-term adherence rates.10–15 To identify subpopulations at greatest risk of therapy discontinuation, this study used administrative data from a large U.S. health plan to describe patient characteristics and comorbidities associated with increased rates of therapy discontinuation during the 2 years after AMI.
METHODS
Design
This is a retrospective observational study examining paid medical and pharmaceutical claims from a large health care organization operating in the Northeastern United States. The study includes claims from indemnity, managed care, and hybrid plans. Participants were enrollees with a hospitalization for AMI between June 1, 2000, and May 31, 2001. Paid medical service claims were used to identify AMI patients. The identification algorithm was similar to the one validated by Kiyota and colleagues.16 The validated claims-based definition of AMI requires a hospitalization episode lasting at least 3 days with an ICD-9-CM of 410.x1 (x = 0–9) listed either as principal or secondary diagnosis. The five-digit ICD-9-CM code 410.x1 represents an initial episode-of-care, and 410.x2 represents a subsequent episode of care, typically involving evaluation or treatment for AMI receiving initial treatment within the preceding 8 weeks. However, in most of the AMI hospitalization claims in our data, the fifth digit was not populated or was coded as 410.x0, corresponding to an unspecified episode-of-care. Therefore, rather than using 410.x1, inpatient claims with ICD-9 = 410 were used to identify AMI encounters, and we studied medical claims histories within the 6 months before each encounter to limit the events to initial hospitalizations. A data set incorporating all paid insurance claims for pharmaceuticals, medical services, and procedures was assembled for these patients, describing encounters within 180 days before and 720 days after initial AMI hospitalization, on services provided both for cardiac care and for all other medical conditions. Participants were enrolled in the insurance plan throughout the study period with prescription drug coverage and had complete enrollment and demographic data.
The study has two separate but partially overlapping sets of subjects because of the makeup of the outcome variables (i.e., discontinuation among those who initiated beta-blocker therapy and discontinuation among those who initiated ACEI/ARB therapy). To analyze beta-blocker discontinuation, participants were limited to enrollees who did not have a contraindication for beta-blockers in their claims histories and those who filled a beta-blocker prescription within 60 days of hospital discharge. As beta-blockers are contraindicated for patients with asthma, we excluded those who filled a prescription for inhaled corticosteroids (beclomethasone, budesonide, flunisolide, fluticasone, and triamcinolone) or had a diagnosis of asthma in their claims histories (ICD-9-CM = 439). In addition, patients with hypotension (ICD-9-CM = 458), heart block >1 degree (ICD-9-CM = 426.0, 426.12, 426.13, 426.2, 426.3, 426.4, 426.51, 426.52, 426.53, 426.54, and 426.7), and sinus bradycardia (ICD-9-CM = 427.81) were excluded. Because chronic obstructive pulmonary disease (COPD; ICD-9-CM = 491.20, 491.21, 492.0, 492.8, 496, 518.1, 518.2, and 506.4) is a relative rather than absolute contraindication, and because these patients initiated beta-blocker treatment, patients with COPD were not excluded, but COPD was controlled for in statistical analyses. The inclusion criteria (other than initiation) yielded 752 patients, of whom 499 initiated beta-blockers within 60 days of discharge. Exclusion criteria for ACEI/ARB analyses were similar to those for beta-blocker analyses except for comorbidities indicating contraindication for beta-blockers; 1,061 patients were identified, of whom 526 (50%) filled an ACEI/ARB prescription within 60 days of discharge.
Outcome Measure
The outcome measure for each cohort was time from initiation to discontinuation. Prescription claims included dispense date, days supplied, and national drug code (NDC) for each filled prescription. Beta-blockers (acebutolol, atenolol, betaxolol, bisoprolol, carteolol, carvedilol, labetalol, metoprolol, nadolol, penbutolol, pindolol, propranolol, sotalol, and timolol), ACEI (benazepril, captopril, enalapril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril, and trandolapril), and ARBs (candesartan, eprosartan, irbesartan, losartan, olmesartan, telmisartan, and valsartan) that were filled by each patient were identified through NDCs on paid claims. Dispense date and days supplied were used to identify the days in which the patient possessed the drug. Therapy was considered discontinued when 60 days or more elapsed after exhausting the cumulative days supplied from prior prescription(s). Inpatient days were excluded when counting towards the 60-day gap. Gaps in therapy of less than 60 days were not treated as “discontinuation”; this approach provides a conservative definition of discontinuation that builds in a “margin for error,” allowing for possibilities such as occasional use of medication samples or billing problems. Medication switching was not classified as discontinuation if there was no extended break in therapy (60 days). Consequently, patients who discontinued an ACEI and started ARB, or switched drugs within the same class, are classified as continuing therapy.
Potential Predictors of Therapy Discontinuation
Patient enrollment information provided data on age, sex, and home address. The median household income of the home address neighborhood was used as a proxy for socioeconomic status.17 Patients were categorized as being from high-income neighborhoods if annual median household income in their ZIP code was higher than $60,000 and lower-income neighborhoods otherwise. Comorbid conditions were identified from the diagnosis codes submitted on medical claims throughout the study period. We identified conditions that increase the risk of secondary AMI or that were highly prevalent, including diabetes (ICD-9-CM = 250), hypertension (ICD-9-CM = 401), dyslipidemia (ICD-9-CM = 272.0–272.4), congestive heart failure (CHF; ICD-9-CM = 428), cerebrovascular disease (ICD-9-CM = 430–438), peripheral vascular disease (ICD-9-CM = 443.9, 785.4, v43.4, 411), chronic kidney disease (ICD-9-CM = 404, 403, 582–583, 585–586, 588), and cancer (ICD-9-CM = 140–172, 174–198, 199.1, 200–208). We also controlled for angina (ICD-9-CM = 413) and coronary artery disease (CAD; ICD-9-CM = 414) that was diagnosed prior to the initial AMI hospitalization. We adjusted for the number of days spent in the hospital for initial AMI care, categorized into 3–7 and 8+ days.
Statistical Analyses
The proportion of persons who continued therapy was graphed across days after initiation (the survival distribution function). Because observations for many people were censored (treatment continued past the last date of observation), survival analysis techniques were used to model time to discontinuation. Survival curves were modeled using the Kaplan–Meier technique, and associations between therapy discontinuation and potential predictors were estimated using Cox proportional hazards regression.18
RESULTS
Beta-Blocker Therapy
Table 1 provides the profile of the cohort that initiated beta-blocker therapy. Two-thirds were male; 59% lived in neighborhoods with lower incomes; 25% were younger than age 55 and more than half were aged 55–74 (54%). The majority of patients (63%) spent 3–7 days in hospital for their initial episode of AMI care. Comorbid conditions were prevalent: 59% had a diagnosis of hypertension during the study period, 39% had dyslipidemia, and 32% had CHF. Prior to initial AMI hospitalization, 27% had a diagnosis of CAD and 8% had angina.
Table 1.
Characteristics of Patients Who Initiated Beta-Blocker Therapy and Factors Associated with Discontinuation
| Cohort that initiated beta-blocker therapy | Discontinuation | ||||
|---|---|---|---|---|---|
| N | % | N | Hazards Ratio [95% CI] | P value | |
| All | 499 | 100 | 237 | ... | ... |
| Sex | |||||
| Female | 166 | 33.3 | 72 | 0.75 [0.56, 1.00] | 0.05 |
| Age | |||||
| ≤55 | 126 | 25.3 | 61 | ... | ... |
| 55–74 | 268 | 53.7 | 123 | 0.90 [0.65, 1.24] | 0.52 |
| >75 | 105 | 21.0 | 53 | 1.08 [0.72, 1.62] | 0.70 |
| Income at the ZIP-code level | |||||
| >60,000 | 206 | 41.3 | 86 | 0.72 [0.55, 0.94] | 0.02 |
| Number of days in hospital | |||||
| 8+ days | 186 | 37.3 | 99 | 1.36 [1.03, 1.80] | 0.04 |
| Comorbid conditions* | |||||
| Hypertension | 294 | 58.9 | 132 | 0.76 [0.58, 0.99] | 0.04 |
| Diabetes | 110 | 22.0 | 58 | 1.13 [0.81, 1.57] | 0.47 |
| Dyslipidemia | 195 | 39.1 | 84 | 0.78 [0.60, 1.04] | 0.09 |
| Prior coronary artery disease | 136 | 27.3 | 66 | 1.10 [0.80, 1.52] | 0.56 |
| CHF | 160 | 32.1 | 82 | 1.05 [0.78, 1.41] | 0.77 |
| Cerebrovascular disease | 137 | 27.5 | 69 | 1.13 [0.84, 1.52] | 0.41 |
| Peripheral vascular disease | 55 | 11.0 | 21 | 0.57 [0.35, 0.92] | 0.02 |
| Prior angina | 41 | 8.2 | 18 | 0.94 [0.56, 1.57] | 0.81 |
| COPD | 48 | 9.6 | 20 | 0.77 [0.48, 1.24] | 0.29 |
| Cancer | 80 | 16.0 | 42 | 1.30 [0.92, 1.83] | 0.13 |
| Kidney disease | 34 | 6.8 | 19 | 1.22 [0.72, 2.09] | 0.46 |
*We created dummy variables indicating presence of each condition. Table 1 presents the subgroups with the comorbidity.
Figure 1 presents the proportion of participants remaining on therapy over the time following treatment initiation. There was an initial drop-off of 6% within the first 30 days, followed by a steady rate of decline in the remainder of the 2-year study period. Even after a year of treatment, the proportion remaining on therapy continued to decline with no clear indication of leveling off. The discontinuation rate reached 18% at 6 months, 28% at 1 year, and 47% at 2 years.
Figure 1.
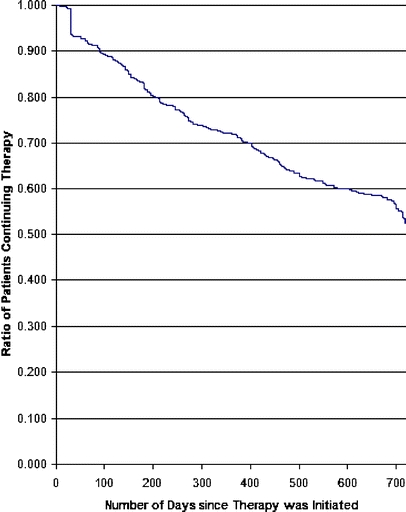
Time to discontinuation for beta-blocker therapy
Table 1 also shows the associations between characteristics of patients and beta-blocker discontinuation (Cox model). Women were less likely to discontinue therapy (25% decrease in dropout hazard; P < 0.05). There were no significant differences in time-to-discontinuation by age. Those living in high-income neighborhoods were less likely to discontinue therapy (28% decrease in dropout hazard; P < 0.05). Compared to patients with shorter stays, those who stayed 8+ days in the hospital were more likely to discontinue therapy (HR = 1.36; P < 0.05). Presence of comorbid hypertension (HR = 0.76; P < 0.05), of peripheral vascular disease (HR = 0.57; P < 0.05), and of dyslipidemia (HR = 0.78; P < 0.10) was associated with lower likelihood of discontinuation.
ACEI/ARB Therapy
The characteristics of the ACEI/ARB cohort are presented in Table 2. Sex and neighborhood income distributions were similar to the beta-blocker cohort; 20% were aged 55 or younger and 57% were aged 55–74. We observed high comorbidity rates: almost two-thirds (64%) had a diagnosis of hypertension during the study period and almost half had a diagnosis of CHF (45%); 26% had comorbid COPD. Before hospitalization for AMI, 34% had a diagnosis of CAD and 12% had angina.
Table 2.
Characteristics of Patients Who Initiated ACEI/ARB Therapy and Factors Associated with Discontinuation
| Cohort that initiated ACEI/ARB therapy | Discontinuation | ||||
|---|---|---|---|---|---|
| N | % | N | Hazards ratio [95% CI] | P value | |
| All | 526 | 100 | 264 | ... | ... |
| Sex | |||||
| Female | 193 | 36.7 | 97 | 0.96 [0.74, 1.24] | 0.76 |
| Age | |||||
| ≤55 | 105 | 20.0 | 53 | ... | ... |
| 55+ | 300 | 57.0 | 139 | 0.80 [0.67, 1.12] | 0.19 |
| >75 | 121 | 23.0 | 72 | 1.21 [0.83, 1.78] | 0.33 |
| Income at the ZIP-code level | |||||
| >60,000 | 217 | 41.2 | 108 | 0.98 [0.76, 1.26] | 0.88 |
| Number of Days in Hospital | |||||
| 8+ days | 212 | 40.3 | 113 | 1.12 [0.86, 1.44] | 0.40 |
| Comorbid conditions* | |||||
| Hypertension | 337 | 64.1 | 172 | 1.10 [0.85, 1.42] | 0.49 |
| Diabetes | 139 | 26.4 | 77 | 1.09 [0.81, 1.45] | 0.55 |
| Dyslipidemia | 207 | 39.4 | 95 | 0.80 [0.61, 1.03] | 0.09 |
| Prior coronary artery disease | 179 | 34.0 | 107 | 1.38 [1.04, 1.83] | 0.02 |
| CHF | 237 | 45.1 | 132 | 1.04 [0.80, 1.37] | 0.75 |
| Cerebrovascular disease | 155 | 29.5 | 76 | 0.79 [0.59, 1.06] | 0.11 |
| Peripheral vascular disease | 75 | 14.3 | 42 | 1.06 [0.75, 1.50] | 0.76 |
| Prior angina | 64 | 12.2 | 44 | 1.51 [1.06, 2.14] | 0.02 |
| COPD | 138 | 26.2 | 80 | 1.30 [0.98, 1.71] | 0.29 |
| Cancer | 82 | 15.6 | 40 | 0.91 [0.64, 1.28] | 0.59 |
| Kidney disease | 52 | 9.9 | 32 | 1.16 [0.77, 1.74] | 0.47 |
*We created dummy variables indicating presence of each condition. Table 2 presents the subgroups with the comorbidity.
Seven percent discontinued therapy after 30 days of initiation (Fig. 2). As with beta-blockers, there was a subsequent steady decline in the proportion remaining on therapy, with no clear indication of leveling off. The discontinuation rate was slightly faster for ACEI/ARBs compared to beta-blockers, with a total of 22% discontinuing within 6 months, 32% within 1 year, and 50% by 24 months.
Figure 2.
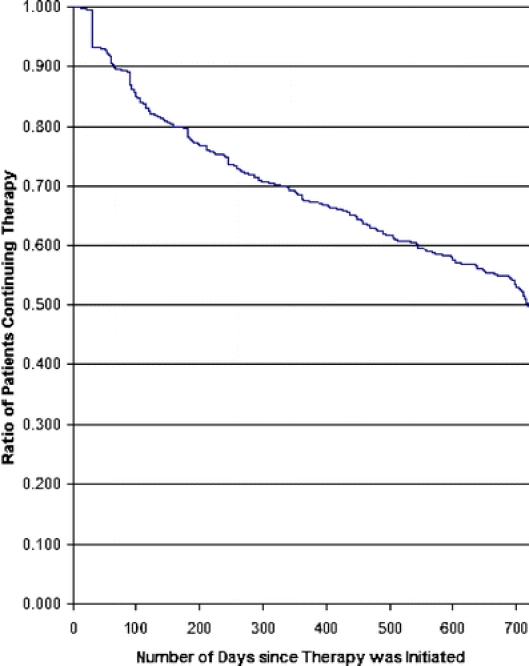
Time to discontinuation for ACEI/ARB therapy
The proportional-hazards model (Table 2) points to variations that are different from those observed for the beta-blocker cohort. In contrast to the beta-blocker cohort, discontinuation of ACEI/ARB therapy was not associated with sex, neighborhood income, or number of hospital days. Presence of CAD during study period (HR = 1.38; P < 0.05) and angina (HR = 1.51; P < 0.05) prior to AMI were significantly associated with higher odds of treatment discontinuation. Similar to the beta-blocker cohort, patients with comorbid dyslipidemia were less likely to discontinue therapy (P < 0.10). The effects of the remaining comorbid conditions were statistically insignificant, including hypertension and peripheral vascular disease, unlike the beta-blocker cohort.
DISCUSSION
Results highlight the difficulty of maintaining consistent long-term use of evidence-based secondary prevention therapies after AMI, even when these therapies are initiated upon hospital discharge. After 2 years of follow up, in a population with continuous health insurance including prescription drug coverage, only about half of AMI patients continuously remained on beta-blocker or ACEI/ARB therapy. Survival analyses suggest that the risk of discontinuation is not limited to those patients with initial difficulties in adjusting and adhering to medication regimens, but continued in a relatively monotonic fashion over the 2-year follow-up period. Even among patients who had successfully remained on the therapies for more than a year post-AMI and were presumably stabilized on these therapies, there was no indication of a “plateau” in continuation rates during the follow-up period. These findings suggest that, to minimize the risk of reinfarction, it is important that support and encouragement to adhere to secondary prevention regimens be provided on an ongoing, long-term basis.
Results also provide some insight into identifying subpopulations at special risk of discontinuation who may be in particular need of support with adherence. Residents of lower-income neighborhoods appeared to be at higher risk of beta-blocker discontinuation. This may not be directly related to the financial burden of medications, as copayments were relatively modest in this population (the copayment was generally $5 for a 3-month supply from a mail-order pharmacy or $5 for a 1-month supply from a retail pharmacy). As there were no gaps in medical or pharmacy coverage and low copayments relative to income, cost of the medication was not expected to be a major barrier to continuous use. Still, despite apparent lack of financial barriers, neighborhood income was a significant factor explaining continuous use of beta-blockers. Understanding the relationship between income and social determinants of health behavior is clearly complex and warrants further investigation. The association between treatment discontinuation and neighborhood income could partially be confounded by race/ethnicity. Many commercial plans do not collect race/ethnicity data. Our results, at minimum, suggest the need for collecting such data to investigate disparities in treatment compliance/adherence.
Patients with certain comorbid conditions (hypertension, dyslipidemia, or peripheral vascular disease) were less likely to discontinue beta-blocker therapy, but other comorbid conditions that increase the risk of secondary AMI (e.g., diabetes or chronic kidney disease) did not significantly predict discontinuation hazard.19,20 While predictors of therapy discontinuation varied between beta-blockers and ACEI/ARB, a common predictive comorbid condition was dyslipidemia. Reported associations may reflect variations in motivation and the perceived need for treatment adherence, which indicates the need for more thorough studies of patient attitudes. Of note, patients with a diagnosis of angina or CAD within 6 months before AMI were more likely to discontinue ACEI/ARB compared to the patients who were free of these conditions before AMI. It is possible that motivation is affected by “rate” of decline in perceived health. AMI patients who were free of prior angina/CAD could perceive the AMI event as a more severe decline in health and, in turn, be relatively more motivated than patients suffering from angina/CAD prior to AMI. In-depth primary data collection is necessary to investigate the mechanisms behind these associations.
Patients who were in the hospital for more than a week were more likely to discontinue therapy compared to those with shorter stays. It is possible that those patients were relatively sicker; some may be experiencing more side effects that adversely effect adherence. The mechanisms behind this association cannot be explained with the current design. However, this variable is readily available for health plan administrators and could be used to identify patients at greater risk for therapy discontinuation. As it is likely to capture relevant clinical variation, we chose to include it in our set of covariates.
We demonstrated that many patients discontinue their therapies after using them for extended periods (e.g., 1 year). Existing studies on outpatient treatment adherence post-AMI are generally limited by relatively short follow-up periods. A few studies extend their follow-up to 1 year.7,9,10,21,22 Yet, in our study population, there is no sign of leveling in the discontinuation rates after 1 year post-AMI. We identified two studies examining adherence beyond the first year post-AMI,8,11 which have limited generalizability, and more importantly, examine patterns from the 1990s, generally focusing on beta-blockers. Our findings reflect treatment patterns in a period of concerted efforts to increase use of beta-blockers and ACEI after AMI. The period was also marked by relatively higher rates of therapy initiation at the time of hospital discharge. Our research makes a unique contribution to the literature by following up patients for 24 months post-AMI in addition to examining the association between socioeconomic status and comorbidities on treatment discontinuation.
Our findings are limited to information derived from insurance claims; clinical details are limited to diagnosis histories and the data lack many relevant variables that could be collected by interviews (e.g., patient’s motivation and side effects experienced). Side effects such as cough and increased potassium can occur on ACEI, and beta-blockers can cause significant fatigue and loss of sexual functioning.23 Even when the patient is well informed about the long-term protective effects of these medications, side effects could counterbalance the patient’s motivation and lead to therapy discontinuation. The outcome measure, pharmacy refill persistence, does not verify administration of drugs but does address the question of drug availability. Possession of a current filled prescription is a necessary, though not always a sufficient condition for therapy adherence. Grymonpre et al. studied the validity of a similar measure for ACEI among elderly individuals and reported 95% concordance with pill counts.24 The high concordance between claims-based measures and pill counts suggests that the rate with which patients refill their medications usually is consistent with the rate with which they consume them.
Inadequate use of these treatments is a significant source of avoidable mortality, morbidity, and consequent health care expenditures. Our results suggest that important improvements are possible in quality of care, and adherence is a priority area for development of better-quality measures and quality-improvement interventions. The National Committee on Quality Assurance recently implemented a relatively longer term measure (6 months) of persistence of beta-blocker treatment after a heart attack.25 In light of our findings of poor therapy persistence, this measure needs to be closely monitored. Our findings also suggest the need for longer-term quality measures because our results indicate that more than half of therapy discontinuation over the first 2 years happens after the initial 6 months of therapy. In addition, quality measures need to be developed for persistent use of ACEI/ARB therapies.
Efforts to improve prescription of appropriate medications upon hospital discharge, which have been a key focus of quality-improvement programs, may not by themselves be sufficient as a means of improving AMI outcomes. Rather, such efforts need to be complemented with sustained efforts to address the difficult challenge of long-term adherence to medication regimens that decrease the risk of reinfarction. More needs to be learned about how to best _target interventions. While disease management strategies often _target patients with the highest overall risk of reinfarction for the most intensive interventions, results for beta-blockers suggest that post-AMI patients with fewer comorbidities may actually have greater risks of treatment discontinuation, perhaps because their involvement with the health care system is less intense and they may have fewer indications for beta-blocker use. Socioeconomic factors also need to be considered in developing interventions to improve adherence.
Acknowledgements
This study was supported in part through Agency for Healthcare Research and Quality grants U18-HS016097 and R24 HS011825; a grant by Horizon Blue Cross Blue Shield of New Jersey; and an Academic Excellence Center grant funded by Rutgers, the State University of New Jersey.
Conflicts of Interest John Bowblis owns stocks of Glaxosmithkline and Pfizer; and is currently doing consulting for Glaxosmithkline. Stephen Crystal owns stocks of Johnson and Johnson, Bristol Myers Squibb, Lilly, and Merck and received honorarium for a lecture from Johnson and Johnson. The other authors reported no conflicts of interest.
References
- 1.National Institutes of Health; National Heart, Lung, and Blood Institute. Morbidity and Mortality: 2004 Chart Book on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD: National Institutes of Health; 2004
- 2.Everly MJ, Heaton PC, Cluxton RJ, Jr. Beta-blocker underuse in secondary prevention of myocardial infarction. Ann Pharmacother. 2004;38(2):286–93. [DOI] [PubMed]
- 3.Rodrigues EJ, Eisenberg MJ, Pilote L. Effects of early and late administration of angiotensin-converting enzyme inhibitors on mortality after myocardial infarction. Am J Med. 2003;115(6):473–9. [DOI] [PubMed]
- 4.Smith SC, Jr, Blair SN, Bonow RO, et al. AHA/ACC scientific statement: AHA/ACC guidelines for preventing heart attack and death in patients with atherosclerotic cardiovascular disease: 2001 update: A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 2001;104(13):1577–9. [DOI] [PubMed]
- 5.Williams SC, Schmaltz SP, Morton DJ, Koss RG, Loeb JM. Quality of care in U.S. hospitals as reflected by standardized measures, 2002–2004. N Engl J Med. 2005;353(3):255–64. [DOI] [PubMed]
- 6.Burwen DR, Galusha DH, Lewis JM, et al. National and state trends in quality of care for acute myocardial infarction between 1994–1995 and 1998–1999: the Medicare health care quality improvement program. Arch Intern Med. 2003;163(12):1430–9. [DOI] [PubMed]
- 7.Butler J, Arbogast PG, BeLue R, et al. Outpatient adherence to beta-blocker therapy after acute myocardial infarction. J Am Coll Cardiol. 2002;40(9):1589–95. [DOI] [PubMed]
- 8.Mitra S, Findley K, Frohnapple D, Mehta JL. Trends in long-term management of survivors of acute myocardial infarction by cardiologists in a government university-affiliated teaching hospital. Clin Cardiol. 2002;25(1):16–8. [DOI] [PMC free article] [PubMed]
- 9.Simpson E, Beck C, Richard H, Eisenberg MJ, Pilote L. Drug prescriptions after acute myocardial infarction: dosage, compliance, and persistence. Am Heart J. 2003;145(3):438–44. [DOI] [PubMed]
- 10.Wei L, Flynn R, Murray GD, MacDonald TM. Use and adherence to beta-blockers for secondary prevention of myocardial infarction: who is not getting the treatment? Pharmacoepidemiol Drug Saf. 2004;13(11):761–6. [DOI] [PubMed]
- 11.Blackburn DF, Dobson RT, Blackburn JL, Wilson TW, Stang MR, Semchuk WM. Adherence to statins, beta-blockers and angiotensin-converting enzyme inhibitors following a first cardiovascular event: a retrospective cohort study. Can J Cardiol. 2005;21(6):485–8. [PubMed]
- 12.Chapman RH, Benner JS, Petrilla AA, et al. Predictors of adherence with antihypertensive and lipid-lowering therapy. Arch Intern Med. 2005;165(10):1147–52. [DOI] [PubMed]
- 13.Eagle KA, Kline-Rogers E, Goodman SG, et al. Adherence to evidence-based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. Am J Med. 2004;117(2):73–81. [DOI] [PubMed]
- 14.Gregoire JP, Moisan J, Guibert R, et al. Determinants of discontinuation of new courses of antihypertensive medications. J Clin Epidemiol. 2002;55(7):728–35. [DOI] [PubMed]
- 15.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113(2):203–12. [DOI] [PubMed]
- 16.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. [DOI] [PubMed]
- 17.United State Census Bureau. Census 2000 Summary File 3 (SF3)—Sample Data. Available at http://factfinder.census.gov/servlet/DatasetMainPageServlet?_program=DEC&_lang=en.
- 18.Allison PD. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC: SAS Institute; 1995.
- 19.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. [DOI] [PubMed]
- 20.Malmberg K, Ryden L, Hamsten A, Herlitz J, Waldenstrom A, Wedel H. Mortality prediction in diabetic patients with myocardial infarction: experiences from the DIGAMI study. Cardiovasc Res. 1997;34(1):248–53. [DOI] [PubMed]
- 21.Kramer JM, Hammill B, Anstrom KJ, et al. National evaluation of adherence to beta-blocker therapy for 1 year after acute myocardial infarction in patients with commercial health insurance. Am Heart J. 2006;152(3):451–58. [DOI] [PubMed]
- 22.LaBresh KA, Ellrodt AG, Gliklich R, Liljestrand J, Peto R. Get with the guidelines for cardiovascular secondary prevention: pilot results. Arch Intern Med. 2004;164(2):203–9. [DOI] [PubMed]
- 23.Goodman LS, Gilman A, Brunton LL, Lazo JS, Parker KL. The Pharmacological Basis of Therapeutics, 11th ed. New York, NY: McGraw-Hill; 2006.
- 24.Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44(5):471–7. [DOI] [PubMed]
- 25.National Committee on Quality Assurance. HEDIS 2005, vol 2: Technical Specifications. Washington, DC: National Committee on Quality Assurance; 2005.


