Abstract
Type 1 diabetes mellitus is associated with a number of disorders of skeletal health, conditions that rely, in part, on dynamic bone formation. A mouse model of distraction osteogenesis was used to study the consequences of streptozotocin-induced diabetes and insulin treatment on bone formation and osteoblastogenesis. In diabetic mice compared with control mice, new bone formation was decreased, and adipogenesis was increased in and around, respectively, the distraction gaps. Although insulin treatment restored bone formation to levels observed in nondiabetic control mice, it failed to significantly decrease adipogenesis. Molecular events altered during de novo bone formation in untreated type 1 diabetes mellitus, yet restored with insulin treatment were examined so as to clarify specific osteogenic genes that may contribute to diabetic bone disease. RNA from distraction gaps was analyzed by gene microarray and quantitative RT-PCR for osteogenic genes of interest. Runt-related transcription factor 2 (RUNX2), and several RUNX2 _target genes, including matrix metalloproteinase-9, Akp2, integrin binding sialoprotein, Dmp1, Col1a2, Phex, Vdr, osteocalcin, and osterix, were all significantly down-regulated in the insulin-deficient, hyperglycemic diabetic animals; however, insulin treatment of diabetic animals significantly restored their expression. Expression of bone morphogenic protein-2, transcriptional coactivator with PDZ-binding motif, and TWIST2, all important regulators of RUNX2, were not impacted by the diabetic condition, suggesting that the defect in osteogenesis resides at the level of RUNX2 expression and its activity. Together, these data demonstrate that insulin and/or glycemic status can regulate osteogenesis in vivo, and systemic insulin therapy can, in large part, rescue the diabetic bone phenotype at the tissue and molecular level.
TYPE 1 DIABETES mellitus (T1DM) is associated with serious skeletal comorbidities, including diminished linear bone growth during the pubertal growth spurt (1,2), early onset osteopenia and osteoporosis (3,4,5,6,7,8), an increased risk of fragility fracture (9,10), as well as poor bone healing and regeneration after injury (9). Data from our laboratory and others examining bone formation and repair in animal models of T1DM have demonstrated that severe deficits in bone formation occur in both spontaneous and induced models of diabetes (11,12,13,14). Moreover, systemic insulin administration has markedly improved bone formation in diabetic animals (12), and even local delivery of insulin to a fracture site has stimulated bone regeneration in the face of systemically unregulated diabetes (11). Although these observational studies have provided consistent findings regarding deficits in bone integrity attributable to the diabetic state, little is known about the specific cellular and molecular mechanisms underlying poor bone formation in T1DM, or through which genetic mediators insulin treatment and/or concomitant improvements in systemic and local glucose homeostasis promote a pro-osteogenic environment.
To study the cellular and molecular consequences of T1DM and insulin treatment on bone regeneration and osteogenesis, we have used a model of direct bone formation using tibial limb lengthening or distraction osteogenesis (DO). Rodent models using DO provide the opportunity to isolate and study osteoblastogenesis, and regenerate intramembranous bone formation under various pathological conditions and on various genetic backgrounds (15,16). DO is a precise methodology by which long bones are severed, and then systematically stretched, to induce bone formation within the expanding gap. In rodent models the bone created within this gap occurs principally via osteoblast-mediated events and does not require cartilaginous scaffolding or endochondral processes. Thus, DO provides a unique model in which to study the actions of systemic or local mediators on osteoblast activity and bone formation. DO in nondiabetic, diabetic, and insulin-treated diabetic mice was used to delineate the impact of T1DM on regenerate bone formation and osteoblastogenesis, and to explore the molecular events underlying this process in vivo.
Materials and Methods
Experimental design
All research protocols were approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences. Female CD-1 mice were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Mice (11–14 wk of age) were injected ip once a day for 5 d with a 40 mg/kg dose of streptozotocin (STZ), inducing diabetes in 88% of the animals within 1 wk. Diabetic animals were alternately assigned to begin treatment with insulin implants (LinBit sustained release insulin implant pellets; LinShin Canada, Inc., Scarborough, Ontario, Canada) or vehicle (blank palmitic acid micro-crystal implants; LinShin Canada, Inc.) at DO surgery. The DO procedure was performed, as described later, on diabetic mice (n = 10) treated with insulin, diabetic mice (n = 11) treated with vehicle, and comparably aged nondiabetic mice (no STZ; n = 11) (study 1). In a second, similarly executed experiment (study 2) intended to supply cellular material from the distraction gaps for gene array analysis, the DO procedure was performed on diabetic mice treated with insulin (n = 6), diabetic mice treated with vehicle (n = 6), and comparably aged nondiabetic mice (n = 7). Plasma glucose values were measured both at surgery and again at killing in all groups as described elsewhere (12). Plasma insulin levels were measured at killing in study 1, using the LINCO Mouse Endocrine Lincoplex assay (MENDO-75K; LINCO Research, Inc., St. Charles, MO).
DO
Mice were anesthetized with sodium pentobarbital (71 mg/kg). Insulin or vehicle implants were inserted under the mid-dorsal skin, using a 12-gauge trocar. A titanium ring fixator was placed on the left tibia, and a mid-diaphyseal osteotomy was created, as previously described (15). The fibula was fractured by direct lateral pressure. The periosteum and dermal tissues were closed, and Buprenex (Reckitt and Coleman Pharmaceuticals, Inc., Richmond, VA; 0.1 mg/kg) was given postoperatively by im injection for analgesia. Distraction was initiated 3 d after surgery at a rate of 0.075 mm twice a day (0.15 mm/d) and continued for 14 d. Mice were killed under anesthesia on postoperative d 17, and the distracted tibiae were harvested for radiographic, histological, and gene array analyses. To have been included as samples for further analyses, tibiae met the following criteria: 1) they were well aligned, 2) they displayed no broken pin sites, 3) no bone chips were present in the gap, and 4) the ankle was intact.
Radiographic analyses of distracted bone by x-ray and micro-computed tomography (μCT)
After at least 48-h fixation in 10% neutral buffered formalin, the left tibiae were removed from the fixators for high-resolution single-beam radiography. A Xerox Micro50 closed system radiography unit (Xerox, Pasadena, CA) was used at 40 kV (3 mA) for 20 sec, and the image was captured on Kodak X-OMAT film (Eastman Kodak Co., Rochester, NY). For quantification, the radiographs were video recorded under low-power magnification (×1.25 objective), and the area and density of mineralized new bone in the distraction gaps were evaluated using National Institutes of Health Image Analysis 1.62 software (National Institutes of Health, Bethesda, MD). The distraction gap was outlined from the outside corners of the two proximal and the two distal cortices, forming a quadrilateral region of interest (ROI). The mineralized new bone area in the distraction gap was determined visually by outlining regions of radio density. The percentage of new mineralized bone within the distraction gap (percent new bone) was calculated by dividing mineralized bone area by the total area of the distraction gap.
Representative specimens of distracted tibiae were determined from the two-dimensional radiographs, and imaged by μCT using a μCT-40 (Scanco, Medical AG, Bassersdorf, Switzerland) and the manufacturer’s software. Approximately 600 contiguous axial (cross sectional) slices, including the entire distraction gap and at least 0.5 mm of both proximal and distal host bone, were obtained at 55 kV and 70 μA, with a voxel size of 12.4 μm in all dimensions. Three-dimensional reconstructions of the distraction gap were generated as described elsewhere (12).
Histological analysis
After distracted tibiae were decalcified in 5% formic acid, dehydrated and embedded in paraffin as previously described (17,18), longitudinal sections (5–7 μm) were stained with hematoxylin and eosin. Sections were selected for analysis to represent a central location within the gap. As detailed previously for x-ray analyses, a quadrilateral ROI was outlined and recorded. New bone was defined as all organized osteoid/sinusoid columns. Both the proximal and distal endosteal new bone matrix were outlined, and the area was recorded. The percentage of new bone area within the DO gap (percent new bone) was calculated by dividing the new bone matrix area by the total distraction gap area.
Analysis of adipocytes and nucleated marrow cells
To investigate the relationship between bone formation and bone marrow function during DO, the adipose tissue and nonadipose tissue in the bone marrow were analyzed and measured from histological hematoxylin and eosin-stained slides that were video recorded under ×100 magnification from nondiabetic mice (n = 6), diabetic mice (n = 5), and diabetic mice treated with insulin (n = 7). For each specimen analyzed, a 0.64-mm2 ROI was selected within the proximal bone marrow adjacent to the distraction gap. Images were analyzed by National Institutes of Health Image J software. Using the gray density threshold icon, the relative area was outlined, and the fat or nonfat nucleated cells (nu cells) were distinguished and measured by visually matching the variable red areas with the fat cells or the nu cells as described elsewhere (19). The numbers of fat cells or nu cells were calculated and expressed as a percentage of total cells in the ROI.
Gene arrays
To investigate the effects of diabetes and insulin treatment on osteogenic gene expression, at the time of being killed (after 14-d distraction), entire distraction gaps containing cellular material from the osteogenic lineage (i.e. stem cells, preosteoblasts, osteoblasts, osteocytes), the sinusoidal lineage (endothelial cells and pericytes), and some osteoclast lineage cell types were harvested from six insulin-treated diabetic mice, six vehicle-treated diabetic mice, and seven nondiabetic mice.
Total RNA was isolated from bone gaps using Tri Reagent (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s suggested protocol. Total RNA was then purified using the RNeasy MinElute Cleanup Kit (QIAGEN, Inc., Valencia, CA) according to the manufacturer’s protocol. Between 0.3 and 2.8 μg total RNA was added to each well of the ReactionReady First Strand cDNA Synthesis Kit (SuperArray no. C-01; SuperArray Biosciences Corp., Frederick, MD). The equivalent of 0.8 μl cDNA was added to each well of the PCR array. Arrays were run on a Bio-Rad iCycler iQ (Bio-Rad Laboratories, Hercules, CA) with the following parameters: cycle 1, 10 min at 95 C; and cycle 2, 15 sec at 95 C, followed by 1 min at 60 C (40 repeats), with optical data collection at 60 C for each repeat. PCR data were analyzed using the RT2 Profiler PCR Array Data Analysis Template provided by the array manufacturer (SuperArray), which uses five housekeeping genes to normalize for variations in input cDNA.
Quantitative real-time PCR
The expression of osteogenic genes whose normalized expression varied significantly among control, diabetic, and insulin-treated mice, as well as other genes of interest, was examined by quantitative real-time PCR using SuperArray primer sets or custom primers designed using Beacon Designer 3.0 (Premier Biosoft Intl., Palo Alto, CA) (Table 1). Reactions, performed in triplicate, contained 250-nm each primer and 5 μl diluted cDNA. Quantitative PCR was performed on an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) with the following parameters: cycle 1, 10 min at 95 C; and cycle 2, 15 sec at 95 C, followed by 1 min at 60 C (40 repeats), and optical data were collected at each 60 C repeat, followed by a melt curve (60–99 C). Quantitation of the expression of each _target mRNA was performed by constructing standard curves, consisting of 5-fold serially diluted cDNA (combined bone gap cDNAs). _target mRNA expression was normalized to actin expression to control for cDNA loading variation.
Table 1.
Primer sets used for quantitative RT-PCR as described in Materials and Methods
| Description | Gene name | Accession no. | Forward primer sequence | Reverse primer sequence |
|---|---|---|---|---|
| Alkaline phosphatase 2 | Akp2 | NM_007431.1 | ggtagattacgctcacaacaac | aggcacagtggtcaaggt |
| Acid-binding protein 2 | Fabp4 | NM_024406.1 | caccgagatttccttcaaactg | cgactttccatcccacttctg |
| Collagen, type VII, α 1 | Col7a1 | NM_007738.2 | gcctgccgcttctctgactg | ctctcacgacgccactccaag |
| Ibsp | Ibsp | NM_008318.1 | gaggaggcaagcgtcactgaag | actggtggcgaggtggtcc |
| MMP-13 | Mmp13 | NM_008607.1 | ttgcgggaatcctgaagaagtc | agtcacctctaagccaaagaaaga |
| MMP-9 | Mmp9 | NM_013599.2 | ccaccacagccaactatgac | tgcccaggaagacgaagg |
| Peroxisome proliferator-activated receptor, γ | Pparg | NM_011146.1 | ggttgacacagagatgccattc | atcacggagaggtccacagag |
| RUNX2 | Runx2 | NM_009820.2 | cgcacgacaaccgcaccat | cagcacggagcacaggaagtt |
| Distal-less homeobox 5 | Dlx5 | NM_005221.5 | ccgtctcaggaatcgccaact | gccgtggtactggtactggtag |
| Osterix (Sp7 transcription factor) | Sp7 | NM_130458.3 | ctcgtctgactgcctgcctag | gcgtggatgcctgccttgta |
| Msh homeobox 1 | Msx1 | NM_010835.2 | ggctgctgctatgacttctttg | cgggcactttgggcttgg |
Statistical analysis
Skeletal parameter comparisons were analyzed using one-way ANOVA. For all other parameters, statistically significant differences between groups were detected using the unpaired Student’s t test. Data are reported as the mean ± sem, and differences were considered statistically significant when P < 0.05. Gene expression was expressed relative to the expression level of the gene of interest in bone gaps isolated from control mice.
Results
Analysis of glucose levels, obtained at the time of being killed, confirmed the condition of hyperglycemia in the STZ-induced diabetic animals; systemic insulin administration lowered serum glucose values, comparable to those observed in control, nondiabetic animals [control: 190.6 ± 6.5 mg/dl; diabetic plus vehicle: 591.2 ± 7.6 mg/dl (P < 0.001 vs. control); and diabetic plus insulin: 182.3 ± 27.4 mg/dl (P < 0.001 vs. diabetic plus vehicle; P = 0.8 vs. control)]. Circulating insulin levels also differed, as expected, among the three groups [control: 93.6 ± 16.2 pm; diabetic plus vehicle: 59.1 ± 2.3 pm (P < 0.05 vs. control); diabetic plus insulin: 223.0 ± 26.4 pm (P < 0.001 vs. diabetic plus vehicle and vs. control)], although the fed or fasting state of each animal at the time of being killed was not determined.
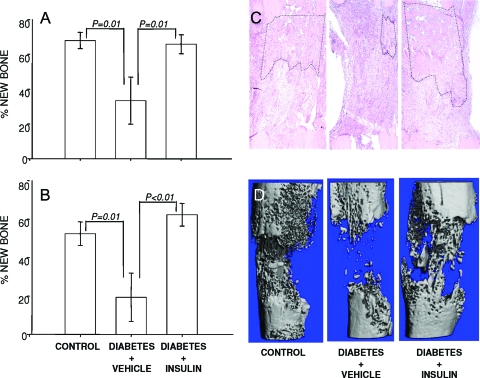
A significant reduction in total bone formation in the distraction gap was noted radiographically (Fig. 1A) in vehicle-treated diabetic animals compared with controls (diabetes plus vehicle: 33.4 ± 13.6% new bone vs. control: 67.9 ± 4.6% new bone). Treatment with systemic insulin restored bone formation to levels that were not statistically different from nondiabetic animals (diabetes plus insulin: 65.8 ± 5.4% new bone). Similarly, histological examination (Fig. 1B) demonstrated significant impairment in bone formation in the vehicle-treated diabetic mice compared with control mice (diabetes plus vehicle: 19.3 ± 12.5% new bone vs. control: 52.4 ± 6.2% new bone), and insulin administration to diabetic animals resulted in markedly improved bone formation (diabetes plus insulin: 62.2 ± 5.9% new bone). Representative histological sections, demonstrated in Fig. 1C, show that bone formation was significantly decreased in the untreated diabetic animal when compared with either a control or insulin-treated diabetic animal. The significant alterations in regenerate bone volume were confirmed when specimens were analyzed for micro-architecture using μCT. Figure 1D illustrates the decrease in bone columns bridging the distraction gap in vehicle-treated diabetic animals when compared with control animals; much of the distorted microarchitecture was improved when diabetic mice were treated with systemic insulin.
Figure 1.
Bone formation is reduced in T1DM. Radiographic (A) and histological (B) assessments of bone formation in control mice, STZ-induced diabetic mice, and insulin-treated diabetic mice are shown. Analyses were performed as described in Materials and Methods. P values for specific between-group comparisons are shown. C, Representative histology sections from distraction gaps of a control mouse, STZ-induced diabetic mouse, and insulin-treated diabetic mouse are presented. New bone formation is outlined in black. D, The effects of diabetes and insulin treatment on bone architecture were assessed by μCT.
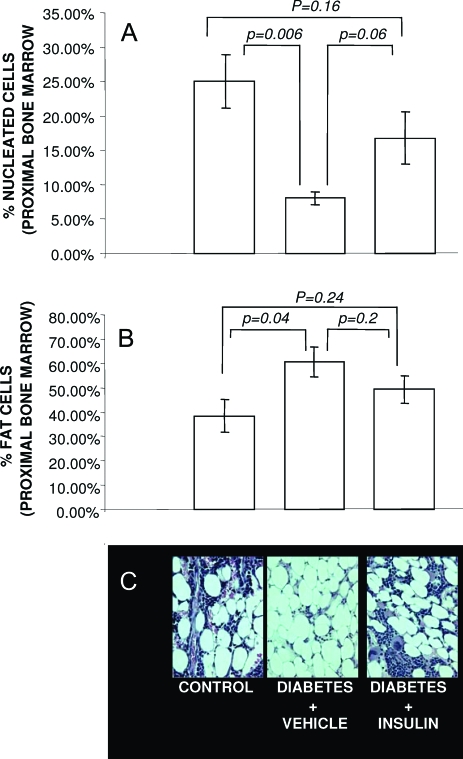
To investigate the potential relationship between bone formation and bone marrow composition during new bone formation, adipocytes and nucleated (nu) cells in the proximal bone marrow were measured in control, vehicle-treated diabetic, and insulin-treated diabetic mice. As shown in Fig. 2A, in the diabetic state, significantly fewer nucleated cells (8.08 ± 0.9%) and a significantly greater number of adipocytes (60.77 ± 6.1%) were identified in the proximal marrow compared with controls (nu: 25.06 ± 3.9%; adipocytes: 38.31 ± 6.8%), suggesting that mesenchymal stem cells were being pushed toward an adipocyte lineage, possibly reducing the pool of osteoblastic precursors available to generate new bone within the distraction gap. Treatment of diabetic mice with insulin increased the number of nucleated cells compared with vehicle-treated diabetic mice (16.8 ± 3.8%; Fig. 2A), yet had only a modest and insignificant impact on decreasing adipocyte number (49.29 ± 5.5%; Fig. 2B).
Figure 2.
Bone marrow adipocyte content is increased in T1DM, and nu cells are decreased. The percentage of nonfat nucleated cells (i.e. nu cells) (A) and adipocytes (B) in bone marrow adjacent to the DO gap was assessed in control animals, STZ-induced diabetic animals, and insulin-treated diabetic animals as described in Materials and Methods. C, Representative micrographs of each condition are shown. P values for specific between-group comparisons are shown.
To identify osteogenic genes regulated in the diabetic state and to determine how insulin administration may impact expression of such genes during intramembranous bone regeneration and repair, distraction gaps of insulin-treated diabetic mice, vehicle-treated diabetic mice, and nondiabetic control mice were harvested, and RNA was prepared for gene arrays. Nylon gene arrays [Oligo GEArray Mouse Osteogenesis Microarray (OMM-026); SuperArray] were used initially, and these arrays demonstrated that several genes were altered in their expression levels in the untreated diabetic state compared with control animals and insulin-treated diabetic animals: matrix metalloproteinase (MMP)-13), MMP-9, integrin binding sialoprotein (Ibsp), and type I collagen (data not shown). However, many genes were considered “absent” based on the thresholds inherent in the analyses, and only a subset was detectable under all conditions examined. Because of the numerous “absent” signals obtained using the nylon arrays, we next used a more sensitive and quantitative osteogenic gene array format [RT2 Profiler PCR Array system, Osteogenesis PCR Array (APM-026); SuperArray]. These arrays provide greater sensitivity and can quantify expression levels using real-time PCR-based techniques; they reliably detect more than 2-fold changes in gene expression. Using samples from four animals from each of the three groups (control, vehicle-treated diabetes, and insulin-treated diabetes), 11 genes were identified as being down-regulated in the distraction gap of untreated diabetic animals; yet, after systemic insulin administration, expression of these genes was comparable between control animals and diabetic animals treated with insulin. Of the 113 genes tested using both nylon and RT2-PCR arrays, 13 genes were identified as being down-regulated in the untreated diabetic animal, whereas only one gene (Procollagen, type VII, α1) was up-regulated, showing that only 12% of the total number of osteogenic genes studied were significantly regulated under these conditions.
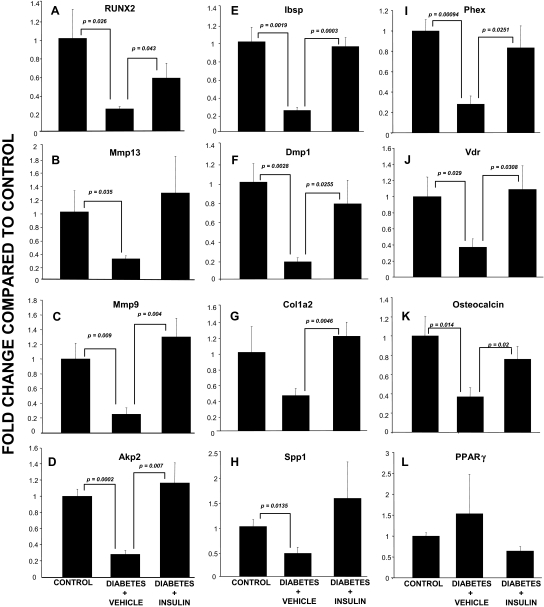
Runt-related transcription factor 2 (RUNX2) by RT2-PCR array was down-regulated in the diabetic animals, yet its expression was normalized when comparing control animals with insulin-treated diabetic animals. Further analyses revealed that the majority of the down-regulated genes identified by nylon array and/or RT2-PCR array have been _targets of RUNX2 [i.e. MMP-9 (20), MMP-13 (21,22), Ibsp (23), collagen (24), Phex (25), DMP-1 (26), alkaline phosphatase (24), and ameloblastin (27)], suggesting that insulin or hyperglycemia may directly or indirectly regulate bone formation through a pro-osteogenic pathway involving RUNX2 expression and RUNX2 downstream _targets. To explore this hypothesis, quantitative RT-PCR (qRT-PCR) was performed for RUNX2 and RUNX2 regulated genes involved in osteogenesis and represented on both nylon and/or RT2-PCR arrays. In addition, other well-characterized _target genes involved in RUNX2 signaling in osteoblasts were examined [i.e. vitamin D receptor (VDR), osteopontin (Spp1), osterix (Sp7), and osteocalcin]. Figure 3, A–K, demonstrate that RUNX2 and all other genes, with the exception of procollagen type I, α2 (G), were significantly down-regulated in the vehicle-treated diabetic animals. Furthermore, RUNX2, MMP-9, Akp2, Ibsp, Dmp1, Col1a2, Phex, Vdr, and osteocalcin were significantly up-regulated when the diabetic animals were treated with insulin, whereas Spp-1 and MMP-13 demonstrated a similar, though not statistically significant, change (Fig. 3). In contrast to the highly modulated gene expression noted for RUNX2 and other pro-osteogenic genes, no significant changes were observed for peroxisome proliferator-activated receptor γ (Fig. 3L) or aP2 (data not shown), which are markers for mature adipocytes; however, this was not entirely surprising because mature adipocytes are only rarely found within the distraction gap (Fig. 1C).
Figure 3.
Gene expression profiles in regenerate bone from control, diabetic, and insulin-treated diabetic mice. Each graph demonstrates the expression level for a gene of interest as assessed by qRT-PCR (described in Materials and Methods) in control, diabetic, and insulin-treated diabetic animals. A, RUNX; B, MMP-1; C, MMP-9; D, Akp2; E, Ibsp; F, Dmp1; G, Colla2; H, Spp1; I, Phex; J, Vdr; K, osteocalcin; L, PPARγ. P values for specific between-group comparisons are shown. PPAR, Peroxisome proliferator-activated receptor.
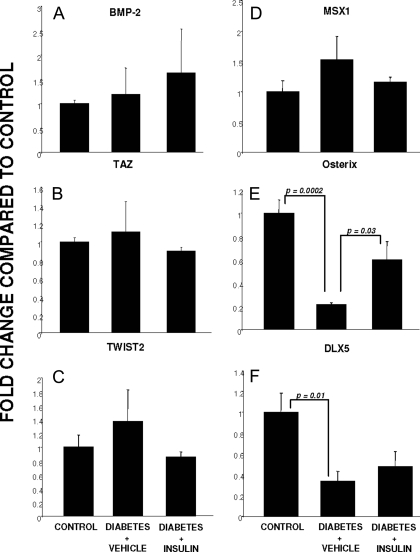
Bone morphogenic protein (BMP)-2 has been a major regulator of RUNX2 and RUNX2-dependent induction of the osteoblast phenotype (28); therefore, its expression was examined in all three conditions. In contrast to RUNX2 expression, BMP-2 expression did not differ among control, diabetic, or insulin-treated diabetic animals (Fig. 4A). Because cells deficient in transcriptional coactivator with PDZ-binding motif (TAZ) have been resistant to BMP-2-induction of the osteoblast phenotype and TAZ is a coactivator of RUNX2 (29), we examined its expression and found no change in its expression in any of the three conditions (Fig. 4B). Similarly, the nuclear inhibitor of RUNX2 in the axial skeleton, TWIST2 (30), was not regulated at the transcriptional level under any of the conditions (Fig. 4C). The transcription factor MSX1 has been suggested to play a role in osteoblast differentiation, especially in the cranium (31). However, its expression was not altered in any of the three conditions studied (Fig. 4D). RUNX2 expression can be regulated upstream by another transcription factor, DLX5, and RUNX2 also regulates DLX5 and osterix production, both important transcription factors regulating genes involved in osteoblast differentiation (32). Osterix expression demonstrated a very similar expression pattern to RUNX2, being significantly down-regulated in the untreated diabetic state, yet up-regulated when insulin was administered systemically (Fig. 4E). As shown in Fig. 4F, DLX5 expression was also significantly down-regulated in the diabetic state. In contrast to RUNX2 and osterix expression, DLX5 expression was not restored in diabetic animals treated with insulin.
Figure 4.
Genes involved in osteoblast commitment and differentiation: expression in regenerate bone from control, diabetic, and insulin-treated diabetic mice. Each graph demonstrates the expression level for a gene of interest as assessed by qRT-PCR (described in Materials and Methods) in control, diabetic, and insulin-treated diabetic animals. A, BMP-2; B, TAZ; C, TWIST2; D, MSX1; E, osterix; F, DLX5. P values for specific between-group comparisons are shown.
Discussion
Similar to our observations using the nonobese diabetic (NOD) mouse model of T1DM (12), these studies demonstrate a significant reduction in new bone formation, assessed both histologically and radiographically, as well as disrupted bone architecture as demonstrated by μCT in STZ-induced diabetes. Because decrements in whole body bone mineral density also occur in both NOD and STZ models (33), these comparisons suggest that the immune mechanisms and cytokine production operative in the NOD model are not necessary for the advent of poor bone regeneration observed in the diabetic state. Thus, the STZ-induced diabetic model provides an opportunity to study the capacity of hyperglycemia and hypoinsulinemia, as well as interventions like insulin treatment, to impact bone pathophysiology in isolation from other autoimmune confounders present in the NOD model.
Our data demonstrate that in vivo, insulin deficiency and hyperglycemia are associated with decreased expression of RUNX2 and downstream _targets of RUNX2, whereas restoration of RUNX2 and RUNX2 regulated genes are observed in regenerate bone obtained from insulin-treated animals. RUNX2 is considered a “master regulator” of osteoblast development, and its expression is essential for normal bone formation (24,34); therefore, factors regulating its expression during osteogenesis may have profound impacts on bone formation. Previous studies have presented conflicting data regarding RUNX2 expression in diabetic bone, suggesting it may (14,35) or may not (33,36) be depressed in skeletal tissues of diabetic animals. Nevertheless, none has used the DO model to study regenerate bone formation in isolation from other events involved in bone formation, turnover, and remodeling. Indeed, the only study to date designed to examine in vivo osteoblastogenesis in diabetes used a bone marrow ablation model wherein there was evidence of early suppression of RUNX2 (14). Our studies show that during in vivo osteoblastogenesis, RUNX2 was significantly down-regulated in the diabetic condition, suggesting that osteoblastogenesis is impaired from the very early stages of osteoblast commitment and may, therefore, explain the profound lack of new bone formation observed in this model. We also demonstrate that insulin treatment can reverse, in large part, the adverse effects that untreated diabetes exerts on RUNX2 expression during osteogenesis.
BMP-2, a critical regulator of osteoblastogenesis and a known mediator of RUNX2 transcriptional activity (28), was not altered in its expression. TAZ, a coactivator of RUNX2, and TWIST2, a cosuppressor of RUNX2, as well as MSX-1, were also not differentially expressed in the diabetic state, suggesting an alternative mode by which RUNX2 and RUNX2 activity might be impaired in diabetic bone. Because MSX-1, TAZ, and TWIST2 are markers of the osteoblast lineage, the finding that they are expressed in distraction gaps from animals with and without diabetes suggests that the cells present within the distraction gap in the diabetic animals likely represent osteoblast precursors that have not progressed normally through the osteogenic programming controlled by RUNX2 and RUNX2 regulated transcription factors, such as DLX5 and osterix (32). Indeed, DLX5, which may function both upstream as well as downstream of RUNX2, was diminished in its expression in the regenerate bone of diabetic animals, as was osterix, suggesting that diminished RUNX2 expression was associated with impairment of other transcription factors that are critical for progression through osteogenic differentiation.
It is difficult to differentiate, within the in vivo situation, the affects of glucose homeostasis from those of insulin status on RUNX2 expression. However, in vitro studies using osteoblast culture systems have shown that extended periods of hyperglycemia do not decrease RUNX2 expression (37). In contrast, insulin has up-regulated the expression of RUNX2 in osteoblastic-like cells in vitro (38,39). Furthermore, recent data suggest that insulin up-regulates RUNX2 in osteoblasts by insulin-receptor mediated events (39). Thus, whereas these in vitro data support a direct role for insulin and insulin signaling in controlling RUNX2 expression and osteoblastogenesis, additional in vivo studies will be required to clarify this possibility.
The consequences of diminished RUNX2 signaling during osteoblastic development are severe and can impact numerous other osteoinductive genes essential to normal osteoblastic function. Complete elimination of RUNX2 signaling results in a profound lack of skeletal components, consistent with its role in both osteogenesis and chondrogenesis (40,41). Recent data comparing gene expression profiles from fetal femurs from wild-type and RUNX2 −/− mice have better clarified which downstream genes are regulated by RUNX2 expression in vivo (25). Of note, genes significantly down-regulated in distraction gaps from diabetic animals in the present study (i.e. Ibsp, MMP-13, MMP-9, Spp-1, Dmp-1, Alk phos, VDR, DLX5, and Phex) were expressed at levels 3-fold (Phex) to more than 2000-fold (Ibsp) higher in wild-type animals compared with RUNX2 −/− animals. Thus, in the diabetic state, there appears to be down-regulation of genes also known to be depressed in genetic RUNX2 deficiency. Each of these RUNX2 _target genes has played essential roles in skeletal homeostasis (reviewed in Ref. 25), and their disruption could have an overall negative impact on bone integrity, particularly on mineralization and mineral homeostasis (Phex, DMP-1, VDR), matrix assembly (Ibsp, Spp-1, DMP-1), and matrix turnover (MMP-9, MMP-13). Indeed, numerous studies together show that diabetic bone demonstrates abnormal mineralization, decreased osteoid surface, and decreased breaking strength (reviewed in Ref. 13).
Our data suggest that in the marrow space adjacent to regenerate bone formation in the untreated diabetic animal, there is evidence of increased adiposity and decreased nucleated precursor cells. Because RUNX2 inhibits the adipogenic phenotype (24), this finding is also consistent with a state of RUNX2 deficiency. In DO, it is believed that precursor cells are recruited from the adjacent marrow and bone elements, resulting in regenerate bone observed within the distraction gap (42); therefore, if precursor cells develop into adipocytes rather than bone-forming cells, new bone formation could be compromised.
In summary, RUNX2, an essential mediator of osteoblastogenesis (24,34), and many genes in the RUNX2 pathway are significantly down-regulated under conditions of hypoinsulinemia and hyperglycemia, yet almost fully restored when insulin is administered systemically. This suggests that RUNX2 may be a primary mediator of diabetes-induced osteopenia, providing an impetus for additional studies to determine better how alterations in insulin and/or glucose homeostasis directly or indirectly regulate RUNX2 expression. Furthermore, this new understanding that the RUNX2 pathway is impaired in diabetic bone suggests that agents that increase RUNX2 activity might be beneficial in ameliorating diabetic bone disease.
Acknowledgments
We thank Drs. James Aronson and Larry Suva for the ability to use the facilities of the Laboratory for Limb Regeneration Research and the Center for Orthopaedic Research, respectively.
Footnotes
This work was supported by Grant SP-030104 (to K.M.T.) from the Children’s University Medical Group fund of the Arkansas Children’s Hospital Research Institute, and in part by National Institutes of Health Grants R01 DK055653 (to J.L.F.), R01AA012223 (to C.K.L.), and C06RR16517 (to Arkansas Children’s Hospital Research Institute).
Present address for D.S.P.: BioMimetic Therapeutics, Inc., Franklin, Tennessee 37067.
First Published Online December 27, 2007
Abbreviations: BMP, Bone morphogenic protein; μCT, micro-computed tomography; DO, distraction osteogenesis; Ibsp, integrin binding sialoprotein; MMP, matrix metalloproteinase; NOD, nonobese diabetic; nu cells, nucleated cells; qRT-PCR, quantitative RT-PCR; ROI, region of interest; RUNX2, runt-related transcription factor 2; STZ, streptozotocin; TAZ, transcriptional coactivator with PDZ-binding motif; T1DM, type 1 diabetes mellitus; VDR, vitamin D receptor.
References
- Ahmed ML, Connors MH, Drayer NM, Jones JS, Dunger DB 1998 Pubertal growth in IDDM is determined by HbA1c levels, sex, and bone age. Diabetes Care [Erratum (1998) 21:1382] 21:831–835 [DOI] [PubMed] [Google Scholar]
- Salerno M, Argenziano A, Di Maio S, Gasparini N, Formicola S, De Filippo G, Tenore A 1997 Pubertal growth, sexual maturation, and final height in children with IDDM. Effects of age at onset and metabolic control. Diabetes Care 20:721–724 [DOI] [PubMed] [Google Scholar]
- Hampson G, Evans C, Petitt RJ, Evans WD, Woodhead SJ, Peters JR, Ralston SH 1998 Bone mineral density, collagen type 1 α 1 genotypes and bone turnover in premenopausal women with diabetes mellitus. Diabetologia 41:1314–1320 [DOI] [PubMed] [Google Scholar]
- Heap J, Murray MA, Miller SC, Jalili T, Moyer-Mileur LJ 2004 Alterations in bone characteristics associated with glycemic control in adolescents with type 1 diabetes mellitus. J Pediatr 144:56–62 [DOI] [PubMed] [Google Scholar]
- Kayath MJ, Dib SA, Vieira JG 1994 Prevalence and magnitude of osteopenia associated with insulin-dependent diabetes mellitus. J Diabetes Complications 8:97–104 [DOI] [PubMed] [Google Scholar]
- Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG 2000 Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest 23:295–303 [DOI] [PubMed] [Google Scholar]
- Lopez-Ibarra PJ, Pastor MM, Escobar-Jimenez F, Pardo MD, Gonzalez AG, Luna JD, Requena ME, Diosdado MA 2001 Bone mineral density at time of clinical diagnosis of adult-onset type 1 diabetes mellitus. Endocr Pract 7:346–351 [DOI] [PubMed] [Google Scholar]
- Tuominen JT, Impivaara O, Puukka P, Ronnemaa T 1999 Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care 22:1196–1200 [DOI] [PubMed] [Google Scholar]
- Loder RT 1988 The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop Relat Res 232:210–216 [PubMed] [Google Scholar]
- Janghorbani M, Feskanich D, Willett WC, Hu F 2006 Prospective study of diabetes and risk of hip fracture: the Nurses’ Health Study. Diabetes Care 29:1573–1578 [DOI] [PubMed] [Google Scholar]
- Gandhi A, Beam HA, O’Connor JP, Parsons JR, Lin SS 2005 The effects of local insulin delivery on diabetic fracture healing. Bone 37:482–490 [DOI] [PubMed] [Google Scholar]
- Thrailkill KM, Liu L, Wahl EC, Bunn RC, Perrien DS, Cockrell GE, Skinner RA, Hogue WR, Carver AA, Fowlkes JL, Aronson J, Lumpkin Jr CK 2005 Bone formation is impaired in a model of type 1 diabetes. Diabetes 54:2875–2881 [DOI] [PubMed] [Google Scholar]
- Thrailkill KM, Lumpkin Jr CK, Bunn RC, Kemp SF, Fowlkes JL 2005 Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab 289:E735–E745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Kraut D, Gerstenfeld LC, Graves DT 2003 Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 144:346–352 [DOI] [PubMed] [Google Scholar]
- Aronson J, Liu L, Liu Z, Gao GG, Perrien DS, Brown EC, Skinner RA, Thomas DM, Morris K, Suva LJ, Badger TM, Lumpkin Jr CK 2002 Decreased endosteal intramembranous bone formation accompanies aging in a mouse model of distraction osteogenesis. E-biomed: J of Regenerative Med 3:7–16 [Google Scholar]
- Einhorn TA 1998 One of nature’s best kept secrets. J Bone Miner Res 13:10–12 [DOI] [PubMed] [Google Scholar]
- Aronson J, Shen XC, Gao GG, Miller F, Quattlebaum T, Skinner RA, Badger TM, Lumpkin Jr CK 1997 Sustained proliferation accompanies distraction osteogenesis in the rat. J Orthop Res 15:563–569 [DOI] [PubMed] [Google Scholar]
- Perrien DS, Brown EC, Aronson J, Skinner RA, Montague DC, Badger TM, Lumpkin Jr CK 2002 Immunohistochemical study of osteopontin expression during distraction osteogenesis in the rat. J Histochem Cytochem 50:567–574 [DOI] [PubMed] [Google Scholar]
- Liu Z, Aronson J, Wahl EC, Liu L, Perrien DS, Kern PA, Fowlkes JL, Thrailkill KM, Bunn RC, Cockrell GE, Skinner RA, Lumpkin Jr CK 2007 A novel rat model for the study of deficits in bone formation in type-2 diabetes. Acta Orthop 78:46–55 [DOI] [PubMed] [Google Scholar]
- Pratap J, Javed A, Languino LR, van Wijnen AJ, Stein JL, Stein GS, Lian JB 2005 The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol 25:8581–8591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy V, Kneissel M, Fournier B, Boyde A, Matthias P 2002 High bone resorption in adult aging transgenic mice overexpressing cbfa1/runx2 in cells of the osteoblastic lineage. Mol Cell Biol 22:6222–6233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamurugan N, Kwok S, Alliston T, Reiss M, Partridge NC 2004 Transforming growth factor-β 1 regulation of collagenase-3 expression in osteoblastic cells by cross-talk between the Smad and MAPK signaling pathways and their components, Smad2 and Runx2. J Biol Chem 279:19327–19334 [DOI] [PubMed] [Google Scholar]
- Vaes BL, Ducy P, Sijbers AM, Hendriks JM, van Someren EP, de Jong NG, van den Heuvel ER, Olijve W, van Zoelen EJ, Dechering KJ 2006 Microarray analysis on Runx2-deficient mouse embryos reveals novel Runx2 functions and _target genes during intramembranous and endochondral bone formation. Bone 39:724–738 [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, van Wijnen AJ, Stein JL, Montecino M, Javed A, Zaidi SK, Young DW, Choi JY, Pockwinse SM 2004 Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 23:4315–4329 [DOI] [PubMed] [Google Scholar]
- Hecht J, Seitz V, Urban M, Wagner F, Robinson PN, Stiege A, Dieterich C, Kornak U, Wilkening U, Brieske N, Zwingman C, Kidess A, Stricker S, Mundlos S 2007 Detection of novel skeletogenesis _target genes by comprehensive analysis of a Runx2(−/−) mouse model. Gene Expr Patterns 7:102–112 [DOI] [PubMed] [Google Scholar]
- Gaikwad JS, Cavender A, D’Souza RN 2001 Identification of tooth-specific downstream _targets of Runx2. Gene 279:91–97 [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Kiyoshima T, Wada H, Matsuo K, Nonaka K, Honda JY, Koyano K, Sakai H 2006 Type II/III Runx2/Cbfa1 is required for tooth germ development. Bone 38:836–844 [DOI] [PubMed] [Google Scholar]
- Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT 2006 BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res 21:637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB 2005 TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309:1074–1078 [DOI] [PubMed] [Google Scholar]
- Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G 2004 A twist code determines the onset of osteoblast differentiation. Dev Cell 6:423–435 [DOI] [PubMed] [Google Scholar]
- Komori T 2006 Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 99:1233–1239 [DOI] [PubMed] [Google Scholar]
- Ryoo HM, Lee MH, Kim YJ 2006 Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene 366:51–57 [DOI] [PubMed] [Google Scholar]
- Botolin S, McCabe LR 2007 Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology 148:198–205 [DOI] [PubMed] [Google Scholar]
- Fulzele K, DiGirolamo DJ, Liu Z, Xu J, Messina JL, Clemens TL, Direct actions of insulin on osteoblasts revealed by Cre-mediated disruption of the IGF-1 receptor. Proc 28th Annual Meeting of the Society for Bone and Mineral Research, Philadelphia, PA, 2006, p 514 (Abstract 1048) [Google Scholar]
- Botolin S, McCabe LR 2006 Inhibition of PPARγ prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol 209:967–976 [DOI] [PubMed] [Google Scholar]
- Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR 2005 Increased bone adiposity and peroxisomal proliferator-activated receptor-γ2 expression in type I diabetic mice. Endocrinology 146:3622–3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botolin S, McCabe LR 2006 Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem 99:411–424 [DOI] [PubMed] [Google Scholar]
- Morsczeck C, Moehl C, Gotz W, Heredia A, Schaffer TE, Eckstein N, Sippel C, Hoffmann KH 2005 In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol Int 29:567–575 [DOI] [PubMed] [Google Scholar]
- Fulzele K, Digirolamo DJ, Liu Z, Xu J, Messina JL, Clemens TL 2007 Disruption of the insulin-like growth factor type 1 receptor in osteoblasts enhances insulin signaling and action. J Biol Chem 282:25649–25658 [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ 1997 Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771 [DOI] [PubMed] [Google Scholar]
- Stricker S, Fundele R, Vortkamp A, Mundlos S 2002 Role of Runx genes in chondrocyte differentiation. Dev Biol 245:95–108 [DOI] [PubMed] [Google Scholar]
- Aronson J 2004 Modulation of distraction osteogenesis in the aged rat by fibroblast growth factor. Clin Orthop Relat Res 425:264–283 [DOI] [PubMed] [Google Scholar]