Abstract
Biomarkers in CSF can offer improved diagnostic accuracy for Alzheimer’s disease (AD). The present study investigated whether the glycoprotein and putative tumor suppressor Dickkopf homolog 3 (Dkk-3) is secreted into CSF and evaluated its applicability as a diagnostic marker for AD. Using our highly specific immunoenzymometric assay, Dkk-3 levels were measured in plasma and/or CSF of patients suffering from depression, mild cognitive impairment (MCI), or AD and compared with healthy subjects. Dkk-3 identity was verified by western blot and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS)/MS. High concentrations of Dkk-3 were detected in CSF compared with plasma (28.2 ± 1.3 vs. 1.22 ± 0.04 nmol/L, respectively). Consistently Dkk-3 expression was demonstrated in neurons of the cortex and epithelial cells of the choroid plexus, the major source of CSF. Significantly increased Dkk-3 levels in plasma and CSF were observed for AD patients compared with healthy subjects but not patients suffering from MCI or depression. In summary, our data indicate that elevated Dkk-3 levels are specifically associated with AD and might serve as a potential non-invasive AD biomarker in plasma.
Keywords: β-amyloid (1–42), Alzheimer’s disease, CSF, Dickkopf-3, p-tau-181, tau
Definitive diagnosis of Alzheimer’s disease (AD) requires both a clinical diagnosis of the disease and postmortem detection of β-amyloid plaques and tau-pathology (McKeel et al. 2004). A probable diagnosis of AD can be established based on clinical criteria, including medical history, physical examination, laboratory tests, neuroimaging, and neuropsychological evaluation (Desai and Grossberg 2005; Fradinger and Bitan 2005). However, early AD, mild cognitive impairment (MCI), and mixed forms of dementia, such as vascular dementia (Bibl et al. 2008), frontotemporal lobe dementia (Bian et al. 2008) or Lewy body dementia (Mollenhauer et al. 2006) are more difficult to diagnose.
Analysis of human body fluids aims to improve the sensitivity and specificity of diagnosing AD and other forms of dementia. To date, three biomarkers have been well established in CSF to diagnose AD: β-amyloid (1–42), total-tau, and phospho-tau-181 (Blennow 2004, 2005). The analysis of CSF is limited because of invasive collection by lumbar puncture. Thus, several studies have been conducted in blood samples to establish specific changes of protein levels. Despite great efforts, so far no specific blood biomarker could be established (Henley et al. 2005; Lewczuk and Wiltfang 2008; Humpel and Marksteiner 2009). However, a recent study reported that the combination of 18 selected biomarkers in plasma may allow the diagnosis of AD with high confidence (Ray et al. 2007).
The secreted glycoprotein Dickkopf homolog 3 (Dkk-3) is the most divergent member of the human Dickkopf family (Krupnik et al. 1999; Niehrs 2006) and in contrast to other family members does not modulate Wnt signaling (Wu et al. 2000; Mao et al. 2001). We have previously reported that Dkk-3 down-regulation in prostate cancer epithelial cells is counterbalanced by a strong up-regulation of Dkk-3 in the blood vessels of the remodeled tissue (Zenzmaier et al. 2008b). This expression in tumor endothelial cells has also been reported for other tumors among them glioma (Untergasser et al. 2008). In the adult mouse forebrain, DKK3 gene expression has been detected by in situ hybridization in the lateral ventricular zone, pyramidal neurons of the hippocampus, and cortical neurons (Diep et al. 2004). Gene expression has also been detected in the cortex and pyramidal cells in the human brain and was reported to be down-regulated in elderly schizophrenic subjects (Ftouh et al. 2005).
In this study, the presence of Dkk-3 and its biochemical nature was evaluated for the first time in CSF. We analyzed if CSF Dkk-3 levels increase by age, as was demonstrated for plasma levels (Zenzmaier et al. 2008a). Furthermore, Dkk-3 plasma and CSF levels of patients suffering from depression, MCI, or AD were compared with healthy subjects using a recently developed sensitive indirect immunoenzymometric assay (IEMA) (Zenzmaier et al. 2008a). We also examined whether CSF levels of tau, phospho-tau-181, and β-amyloid (1–42) correlate to changes in Dkk-3 levels, especially for AD.
Materials and methods
Diagnosis of AD, MCI, and geriatric depression
Healthy control subjects and patients were recruited at the Memory Clinics of the Department of Psychiatry in Innsbruck, Austria. Study participants were assessed by identical diagnostic procedures. Psychiatrists clinically examined all subjects, performed a standardized neurological examination, reviewed medical records, and conferred with referring physicians for all patients. All subjects underwent neuropsychological assessments and magnetic resonance neuroimaging. In general, only patients who fulfilled diagnostic criteria for MCI, AD, or depression were included. Healthy subjects had no cognitive impairment. Patients and control subjects were excluded when there were clinical signs of infection or when laboratory testing indicated an ongoing infection. MCI was diagnosed according to the criteria of Petersen et al. (2001). Probable AD was diagnosed according to National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al. 1984). Geriatric depression was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria. No financial remuneration was provided for study participation. The study was approved by the local ethical committee.
Collection of CSF and detection of β-amyloid (1–42), tau, and phospho-tau-181
CSF was collected during routine analysis for measurement of β-amyloid (1–42), tau, and phospho-tau-181. CSF was obtained by lumbar puncture, collected in polypropylene tubes, and frozen at −80°C not later than 3 days after collection. Analysis of β-amyloid (1–42), total-tau, and phospho-tau-181 in CSF was performed by a commercially available ELISA from Innogenetics (NV, Gent, Belgium) as described previously (Blasko et al. 2006). Only samples with a phospho-tau-181/β-amyloid (1–42) ratio > 70 were included for AD, a ratio < 3 for controls and a ratio between 20 and 40 for MCI (Blasko et al. 2006).
Collection of plasma
A total of 10 mL EDTA blood was collected and was processed within 90 min. Samples were centrifuged (400 g, 30 min) on a Biocoll (Biochrom, Berlin, Germany) gradient and the upper plasma phase was immediately frozen at −80°C.
Identification of Dkk-3 in CSF by western blot and MALDI-TOF MS/MS
CSF and recombinant Dkk-3 were separated on a 4–20% gradient Tris–glycine gel (PAGEr® Duramide® Precast Gels; Cambrex, East Rutherford, NJ, USA) and transferred to a Immun-Blot™ polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, Vienna, Austria). Membranes were probed with our highly specific mouse monoclonal antibody for Dkk-3 (mAb; Code: INN-Dkk3-1) at a dilution of 1: 2000 (Zenzmaier et al. 2008a). Direct competition on the membrane with a 50-fold excess of recombinant human Dkk-3 (recDkk-3) served as a specificity control. Detection was performed with horseradish peroxidase-conjugated secondary antibodies (Promega, Mannheim, Germany), chemiluminescent substrate (Amersham ECL™ Western Blotting Analysis System; GE Healthcare, Vienna, Austria) and exposure to enhanced chemiluminescence Hyperfilm (GE Healthcare).
For immunoprecipitation, 250 μL of CSF was diluted 1: 5 with modified radioimmunoprecipitation assay (RIPA) buffer [10 mmol/L Tris–HCl, pH 7.4; 150 mmol/L NaCl; 1% NP-40, (Sigma, Vienna, Austria); 0.25% Na-deoxycholate; and complete Mini Protease Inhibitor Cocktail (Roche Diagnostics, Vienna, Austria)] and 1 μL mAb INN-Dkk3-1 (8 mg/mL) were added. The mixture was incubated overnight at 4°C on a rotary shaker. A Protein G-agarose resin (Upstate, Lake Placid, NY, USA) was then added and the sample was incubated for further 2 h at 4°C. Subsequently, the probe was centrifuged (16 000 g, 10 min), supernatant was removed, and Protein G–agarose resin was washed five times with modified RIPA buffer. Protein G–agarose resin was heated to 95°C for 10 min in 50 μL modified RIPA buffer and centrifuged at 16 000 g for 10 min. Dkk-3 containing supernatant was separated on a 4–20% gradient Tris–glycine gel (PAGEr® Duramide® Precast Gels; Cambrex) and proteins were stained with a Coomassie Brilliant Blue G-250 solution (PageBlue™ Protein Staining Solution; Fermentas, St. Leon-Rot, Germany).
The protein band at the size of Dkk-3 was excised from the gel and chopped into pieces of about 1 × 1 mm. After reduction with 10 mmol/L dithiothreitol and alkylation by 50 mmol/L iodoacetamide, the sample was digested with trypsin, and subsequently the extracted peptides analyzed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) at the Protein Mircoanalysis Facility of the Innsbruck Medical University. Mass spectrometry (MS)/MS spectra were searched against a human database using mascot (Matrix Science, Boston, MA, USA).
Tissue samples
The brain of a 54-year-old man, with no known neurological or psychiatric disease, was obtained at routine autopsy at a postmortem interval of 12 h. Hospital and other medical records confirmed normal intellectual function until the time of death. Histological examination revealed no neuropathological features.
Tissue samples were taken from the frontal, the temporal, the parietal and occipital cortex, and the hippocampus. Tissue blocks were divided midsagittally. The left half was sectioned in 8 mm coronal slabs, and immediately fixed by immersion in cold 4% p-formaldehyde in sodium phosphate buffer (PBS), pH 7.2, for 1 week. Blocks were either dehydrated in graded ethanol, embedded in paraffin, and cut serially in 3 μm thin coronal sections or frozen in isopentane (−55°C) and stored at −70°C until use.
Immunohistochemistry
Paraffin-embedded tissue sections were deparaffinized and rehydrated through Roti®-histol (Carl Roth GmbH, Karlsruhe, Germany) and a graded ethanol series. Thereafter, antigen retrieval was performed by microwave treatment in citrate-buffer (10 mmol/L, pH 6.0) and endogenous peroxidase activity blocked using 3% H2O2/methanol. Sections were incubated for 45 min in blocking solution containing 10% rabbit serum and then stained overnight at 4°C with mouse mAb INN-Dkk3-1 (1.0 μg/mL). Primary antibodies were detected following incubation with a biotinylated rabbit anti-mouse IgG (DAKO Cytomation, Vienna, Austria) using the FAST DAB Tablet Set (Sigma). Sections were counterstained with Mayer’s Hemalum and mounted with Entellan (Merck, Darmstadt, Germany). Specificity controls of the mAb were performed by blocking experiments with 50-fold excess of recDkk-3. Cross-reactivities toward the homologous recombinant proteins Dkk-1, Dkk-4, and Soggy (R&D Systems, Minneapolis, MN, USA) were determined by radioimmunoassays to be ≫ 0.1% (data not shown).
Dkk-3 immunoenzymometric assay
Dkk-3 IEMA was performed as previously described (Zenzmaier et al. 2008a). In brief, 96-well plates were coated with 4 μg/mL primary HPLC-purified mAb INN-Dkk3-1. After a blocking step with 1% bovine serum albumin (BSA)/PBS wells were incubated with antigen overnight at 4°C. After washing plates were incubated with 200 ng/mL of biotinylated polyclonal goat anti-Dkk-3 antibody (Cat. # BAF1118; R&D Systems) in 1% BSA/PBS for 2 h at 25°C. Signals were recorded after incubation with streptavidin/horseradish peroxidase (1: 500 in 1% BSA/PBS; DAKO Cytomation) and the substrate tetramethylbenzidine/H2O2 (Substrate Reagent Pack; R&D Systems) with a Victor2 1420 multilabel counter (Wallac, Freiburg, Germany). For measurement of Dkk-3 plasma samples were diluted 1: 40, CSF samples 1: 1000 in 1% BSA/PBS. All samples were run in duplicate.
Statistical analyses
Results are expressed as mean values ± SEM. Statistical differences among groups were calculated by unpaired Student’s t-test and regarded significant when p < 0.05.
The ability of Dkk-3 levels, β-amyloid (1–42) levels, and β-amyloid (1–42)/Dkk-3 ratios to predict MCI or AD was assessed by receiver operating characteristics (ROC). Area under the ROC curve (AUC) was calculated using rockit software (Kurt Rossmann Laboratories, University of Chicago, Chicago, IL, USA).
Results
High levels of Dkk-3 in CSF
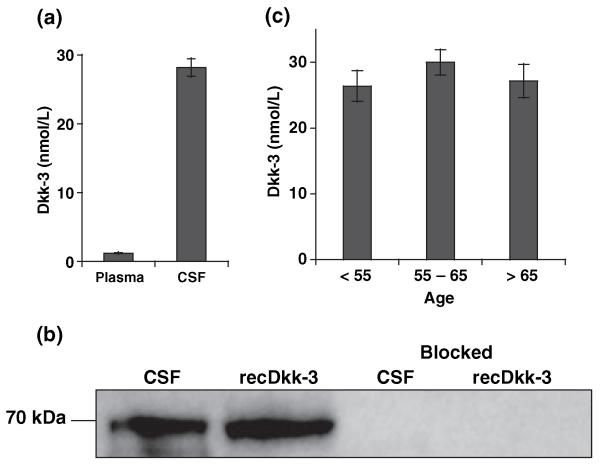
Experiments were set up to address the question if Dkk-3 was present at all in CSF. Thus, protein levels were determined by IEMA in CSF from 26 and in plasma of 25 healthy subjects. Analyses revealed the presence of high levels of Dkk-3 in CSF (28.2 ± 1.3 vs. 1.22 ± 0.04 nmol/L in plasma; Fig. 1a). The biochemical nature of Dkk-3 derived from CSF was verified by comparing it to recDkk-3 (Zenzmaier et al. 2008b) in western blot analysis by mAbs. Proteins from both sources migrated as ~70 kDa band in sodium dodecyl sulfate–polyacrylamide gel electrophoresis indicating an identical degree of glycosylation (the theoretical molecular weight of the unglycosylated protein is 36.2 kDa). The intensity of the Dkk-3 band was comparable to an equal amount of recDkk-3 confirming the high concentrations in CSF measured by IEMA (Fig. 1b). Additionally, the nature of Dkk-3 in CSF was verified by MS after immunoprecipitation (Table 1).
Fig. 1.
High levels of Dkk-3 in CSF. (a) Dkk-3 levels of control probands were ~25-fold higher in CSF (28.2 ± 1.3 nmol/L; n = 26) compared with plasma (1.21 ± 0.04 nmol/L; n = 25). (b). A total of 2 μL of CSF (containing ~80 pmol Dkk-3) and 80 pmol of recDkk-3 were analyzed by western blotting. Dkk-3 in CSF equals the recombinant protein in size (~70 kDa) and signal intensity. Signals in both samples can be completely blocked by direct competition with 50-fold excess of recDkk-3, demonstrating the specificity of our mAb (Zenzmaier et al. 2008a). (c) Dkk-3 content of CSF did not change significantly with age, when comparing control individuals < 55 years (26.4 ± 2.3 nmol/L; n = 7), 55–65 years (30.0 ± 1.9 nmol/L; n = 11), and > 65 years (27.2 ± 2.5 nmol/L; n = 8).
Table 1.
MS/MS analysis of Dkk-3 isolated from CSF
| Sequence | MH+ | Mass (%) | Position | AA (%) |
|---|---|---|---|---|
| GLLFPVCTPLPVEGELCHDPASR | 2564.35 | 7.09 | 204–226 | 6.69 |
| LLDLITWELEPDGALDR | 1969.13 | 5.44 | 227–243 | 4.86 |
| EVPDEYEVGSFMEEVR | 1914.93 | 5.29 | 281–297 | 4.86 |
| Total | 6448.41 | 17.82 | 54 | 16.41 |
AA, amino acid; MS, mass spectrometry; Dkk, Dickkopf homolog. The identified AA sequences after chymotryptic digestion, the 1+ charge state [(M + H)+] of the Dkk-3 protein fragments, the percent mass, the positions of amino acid residues, and percentage of the AA sequence (%) obtained by database search using MASCOT are shown. The mature Dkk-3 protein without signal sequence was taken as the basis for analysis.
CSF donors were divided into three groups according to age (< 55 years, n = 7; 55–65 years, n = 11; and > 65 years, n = 8) and Dkk-3 levels compared in order to detect possible age-related changes. In contrast to plasma (Zenzmaier et al. 2008a), CSF Dkk-3 values were not altered significantly by age (26.4 ± 2.3, 30.0 ± 1.9, and 27.2 ± 2.5 nmol/L for the single age cohorts; Fig. 1c).
Dkk-3 is expressed in cortex and epithelial cells of the choroid plexus
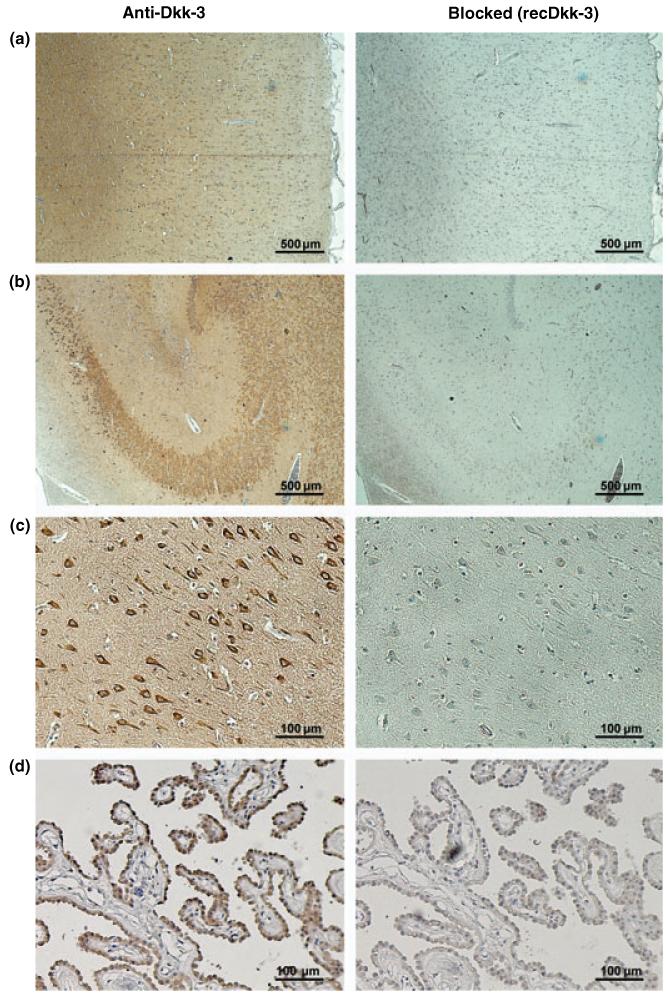
As the source of the high Dkk-3 levels in CSF is yet unknown, brain tissue sections were probed for Dkk-3 with our highly specific mouse mAb. Sections from areas of the frontal, the temporal, and the parietal and occipital cortex showed strong Dkk-3 expression in neurons, in particular pyramidal cells (Fig. 2a). Blocking experiments with an excess of recDkk-3 demonstrated specificity of the signal. In the hippocampus, signals were observed mainly in the Ammon’s horn, where pyramidal cells as well as mossy fibers stained strongly positive for Dkk-3 (Fig. 2b and c).
Fig. 2.
Localization of Dkk-3 in brain tissue. Areas of the frontal cortex (a), the hippocampus (b and c) and the choroid plexus were immunohistochemically probed for Dkk-3 and counterstained with Mayer’s Hemalum. Specificity controls of the mouse mAb Dkk3-1 were performed by blocking experiments with an excess of recDkk-3 (right panel). Strong signals for Dkk-3 were detected in neurons of the isocortex and in the Ammon’s horn of the hippocampus. In the later, Dkk-3 was mainly expressed in pyramidal cells (c: detail of Cornu Ammonis area CA2). Furthermore, Dkk-3 was strongly expressed in epithelial cells of the choroid plexus. Original magnifications: (a and b), 40× and (c and d), 200×.
Additionally to areas from the iso- and allocortex, the choroid plexus, the major source of CSF, was probed for Dkk-3. The epithelial cells of the tissue showed strong Dkk-3 expression, indicating secretion of the protein from these cells into CSF (Fig. 2d). Again signals were blocked by recombinant protein to show specificity.
Elevated Dkk-3 plasma levels in patients with Alzheimer’s disease
To elucidate disease-associated changes of Dkk-3 blood levels, plasma samples of 15 depression, 25 MCI, and 25 AD patients were evaluated by IEMA and compared with the control probands. Depressed patients had a slightly but not significantly reduced mean Dkk-3 plasma level (1.13 ± 0.06 vs. 1.22 ± 0.04 nmol/L). While the protein levels in MCI patients remained unchanged (1.23 ± 0.05 nmol/L), levels were significantly increased in patients with AD (1.33 ± 0.04 nmol/L). To exclude artifacts from the previously described age-associated increase of Dkk-3 levels in plasma of healthy elderly (Zenzmaier et al. 2008a) only subjects at ages above 60 years were included in the analysis. The age characteristics and mean Dkk-3 values of the single cohorts are summarized in Table 2.
Table 2.
Dkk-3 plasma levels of controls, depression, MCI, and AD patients
| Age characteristics (years) |
Dkk-3 plasma levels (nmol/L) |
||||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SEM | Median | Range | Mean ± SEM | Median | Range | |
| Control | 25 | 70.6 ± 1.2 | 69 | 61–80 | 1.22 ± 0.04 | 1.22 | 0.89–1.87 |
| Depression | 15 | 67.7 ± 1.5 | 67 | 60–82 | 1.13 ± 0.06 | 1.14 | 0.79–1.52 |
| MCI | 25 | 72.9 ± 1.7 | 70 | 62–90 | 1.23 ± 0.05 | 1.24 | 0.67–1.83 |
| AD | 25 | 78.5 ± 1.4 | 80 | 64–90 | 1.33 ± 0.04* | 1.36 | 0.96–1.88 |
AD, Alzheimer’s disease; MCI, mild cognitive impairment; Dkk, Dickkopf homolog. Statistical significance versus controls was determined by unpaired two-sided Student’s t-test
p < 0.05.
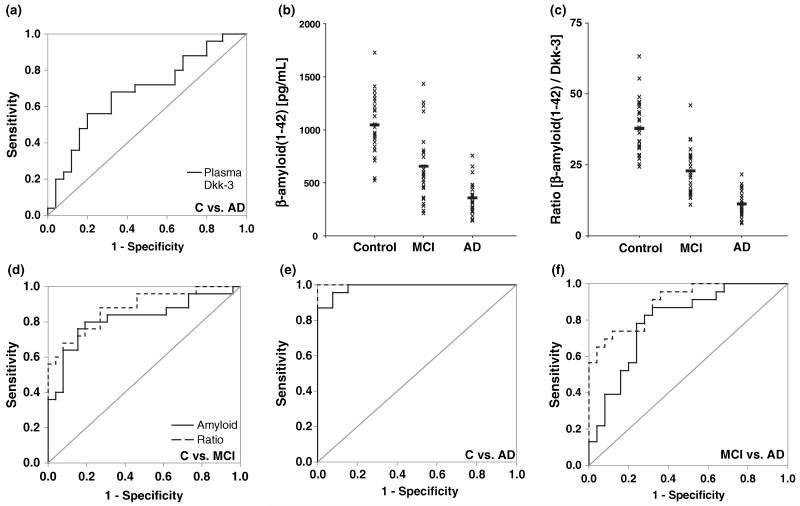
To assess the applicability of Dkk-3 plasma levels as a classifier for AD, ROC analysis was performed (Fig. 3a). The calculated accuracy (AUC = 0.691) indicated fair sensitivity and specificity for Dkk-3 levels to discriminate AD patients from control subjects.
Fig. 3.
Dkk-3 and β-amyloid (1–42) levels as classifiers for diagnosis. (a) ROC curve for the classification of AD patients versus control subjects based on Dkk-3 plasma levels. (b) A one-dimensional scatter plot of β-amyloid (1–42) levels (individual levels marked with ‘x’; mean values with ‘–’) revealed high heterogeneity of the single patient cohorts. (c) A one-dimensional scatter plot of the ratio of β-amyloid (1–42) levels/Dkk-3 levels showed less heterogeneity of the single patient cohorts. (d–e) ROC curves for the classification of (d) MCI and (e) AD patients versus control subjects and (f) MCI versus AD patients based on β-amyloid (1–42) levels and the ratio β-amyloid (1–42)/Dkk-3, respectively.
Elevated Dkk-3 CSF levels in patients with Alzheimer’s disease
CSF Dkk-3 levels from 25 MCI and 23 AD patients were determined by IEMA and compared with the control group. Dkk-3 values of MCI patients were slightly but not significantly increased (30.6 ± 2.8 vs. 28.2 ± 1.3 nmol/L). Like in plasma, the levels of the glycoprotein were significantly elevated in the CSF of patients with AD (33.6 ± 2.2 nmol/L). Patients age characteristics and CSF Dkk-3 levels are given in Table 3. The mean age of the control group was significantly lower compared with the patient cohorts with MCI and AD; however, as Dkk-3 levels in CSF do not alter with age (Fig. 1c) this difference in chronological age could not be causative for the specific increase in Dkk-3 of AD patients.
Table 3.
Dkk-3, tau, phospho-tau-181, and β-amyloid (1–42) levels in CSF of controls, MCI, and AD patients
| Control (n = 26) |
MCI (n = 25) |
AD (n = 23) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Median | Range | Mean ± SEM | Median | Range | Mean ± SEM | Median | Range | |
| Age [years] | 58.9 ± 2.3 | 62 | 32–82 | 68.5 ± 2.1 | 68 | 46–86 | 73.3 ± 1.9 | 74 | 53–89 |
| Dkk-3 [nmol/L] | 28.2 ± 1.3 | 27.4 | 18.6–42.1 | 30.6 ± 2.8 | 28.2 | 4.7–55.1 | 33.6 ± 2.2* | 30.9 | 20.3–65.6 |
| Total-tau [pg/mL] | 235 ± 18 | 232 | 46–441 | 433 ± 62** | 340 | 45–1200 | 797 ± 49*** | 794 | 509–1358 |
| Phospho-tau-181 [pg/mL] | 34 ± 3 | 32 | 16–69 | 54 ± 6** | 49 | 10–109 | 96 ± 7*** | 99 | 36–156 |
| β-Amyloid (1–42) [pg/mL] | 1045 ± 55 | 1038 | 523–1728 | 655 ± 66*** | 585 | 215–1434 | 357 ± 32*** | 324 | 144–757 |
| Ratioa | 37.8 ± 2.0 | 36.8 | 24.4–63.3 | 22.8 ± 1.7*** | 21.4 | 10.9–46.0 | 11.2 ± 1.0*** | 10.0 | 4.4–21.6 |
AD, Alzheimer’s disease; MCI, mild cognitive impairment; Dkk, Dickkopf homolog
β-amyloid (1–42)/Dkk-3. Statistical significance versus controls was determined by unpaired two-sided Student’s t-test
p < 0.05
p < 0.01
p < 0.001.
Dkk-3 correlates with tau and phospho-tau-181 levels in CSF
CSF levels of total-tau and phospho-tau-181 were significantly increased in patients with MCI and significantly further increased in patients with AD (Table 3). To analyze correlations between Dkk-3 and tau levels, the individuals of each cohort (Control, MCI, and AD) were classified into three commensurate groups according to their total-tau levels. In all cohorts with increasing total-tau levels also Dkk-3 levels increased revealing positive correlations of the proteins (Dkk-3 levels [nmol/L]: Control: 21.9 ± 1.7 [total-tau < 150 pg/mL], 29.1 ± 1.9 [150–250], 30.2 ± 2.0 [> 250]; MCI: 18.3 ± 3.8 [< 200], 30.9 ± 4.5 [200–500], 38.5 ± 3.6 [> 500]; AD: 28.5 ± 2.1 [< 650], 31.2 ± 2.9 [650–850], 40.7 ± 4.5 [> 850]). The same analysis was performed for phospho-tau-181 and again revealed positive correlations between Dkk-3 and phospho-tau-181 in all three cohorts (Dkk-3 levels [nmol/L]: Control: 22.9 ± 1.5 [phospho-tau-181 < 20 pg/mL], 27.7 ± 1.4 [20–40], 32.8 ± 2.4 [> 40]; MCI: 17.3 ± 3.7 [< 30], 30.6 ± 4.0 [30–60], 38.6 ± 3.8 [> 60]; AD: 25.8 ± 2.2 [< 70], 33.4 ± 2.2 [70–100], 37.6 ± 4.2 [> 100]).
Dkk-3 and the ratio β-amyloid (1–42)/Dkk-3 as classifiers for diagnosis
In contrast to Dkk-3, CSF levels of β-amyloid (1–42) were significantly decreased in patients with MCI and significantly further reduced in patients with AD (Table 3).
A one-dimensional scatter plot of the β-amyloid (1–42) levels showed a high heterogeneity of the individual values in the three cohorts (Fig. 3b). Given the negative correlation between Dkk-3 and β-amyloid (1–42) we analyzed the ratio of β-amyloid (1–42)/Dkk-3 (Table 3). A one-dimensional scatter plot of this ratio showed reduced heterogeneity compared with β-amyloid (1–42) levels alone (Fig. 3c), suggesting the use of the β-amyloid (1–42)/Dkk-3 ratio instead of β-amyloid (1–42) levels to differentiate between controls, MCI and AD patients.
To further substantiate these findings the accuracy of the β-amyloid (1–42)/Dkk-3 ratio in comparison to β-amyloid (1–42) to discriminate the single cohorts was assessed by ROC analysis. The ability to segregate between two cohorts was increased using the ratio in all cases, control subjects versus MCI patients (Fig. 3d; β-amyloid (1–42): AUC = 0.817; ratio: AUC = 0.894), control subjects versus AD patients (Fig. 3e; β-amyloid (1–42): AUC = 0.981; ratio: AUC = 1.0) and MCI versus AD patients (Fig. 3f; β-amyloid (1–42): AUC = 0.812; ratio: AUC = 0.902).
Discussion
Dkk-3 in CSF of controls, MCI, and Alzheimer’s disease patients
To our knowledge, this is the first report of Dkk-3 in human CSF. Our data demonstrate that Dkk-3 is present in CSF at high concentrations (28.2 ± 1.3 nmol/L) compared with plasma (1.22 ± 0.04 nmol/L). Given the fact that CSF mainly represents an ultrafiltrate of plasma and therefore the total protein concentration is highly reduced (0.15–0.45 vs. 60–80 mg/mL in plasma) the high content of Dkk-3 is even more astonishing. This high concentration of Dkk-3 in CSF indicates an important function of the protein in the brain/CSF compartment. Of all body fluids we tested, Dkk-3 levels are by far highest in CSF [Dkk-3 levels in seminal fluid are similar or higher to that in plasma (2.59±0.41 nmol/L; range 1.62–5.25 nmol/L; n = 10), while levels of Dkk-3 were below detection limit in urine (< 5 pmol/L; n = 3)].
In contrast to plasma Dkk-3 levels, which increase with age (Zenzmaier et al. 2008a), Dkk-3 levels in CSF did not alter significantly with age as shown in the present study. However, because of the lack of CSF samples from younger patients, we could not include a cohort of young adults (age 20–30 years).
Because of the high Dkk-3 content and the proximity to the diseased tissue, we hypothesized CSF might represent a valuable source to trace changes in Dkk-3 levels associated with neurodegenerative disorders. Thus, CSF samples from patients suffering from MCI and AD were analyzed and compared with healthy controls. Indeed, a significant elevation of Dkk-3 levels in AD patients was observed, indicating a potential role of the protein in the development of the disease and its use for diagnostic purposes.
Dkk-3 levels in plasma of controls, depressed, MCI, and AD patients
Similar to CSF, Dkk-3 levels in plasma of patients suffering from AD, but not MCI or depression, was significantly elevated compared with healthy controls. However, in this study, we did not differentiate between MCI subtypes. Most of the patients with amnestic MCI convert to AD (Jicha et al. 2006). Further studies for amnestic MCI patients compared with patients with other MCI subtypes should reveal whether Dkk-3 levels differ among MCI subgroups, and these studies will clarify to which extent amnestic MCI patients are similar to AD patients.
The origin of the Dkk-3 increase in plasma of AD patients is not resolved. One source could be endothelial cells where Dkk-3 is reported to be expressed (Kupatt et al. 2005; Goodwin et al. 2006). Furthermore, up-regulation of Dkk-3 in endothelial cells has been demonstrated in various tumor tissues (St Croix et al. 2000; Untergasser et al. 2008; Zenzmaier et al. 2008b). High expression of the protein has also been reported in a subset of adult human pancreatic beta cells (Hermann et al. 2007).
Given the high concentration of Dkk-3 in CSF, a major source of Dkk-3 in plasma might also be resorption of CSF. This hypothesis is further supported by the fact that the AD-related elevation of Dkk-3 is to a similar extend in both body fluids.
Potential sources of CSF Dickkopf homolog-3
There are several potential sources for the high Dkk-3 levels in CSF. CSF is mainly produced in the choroid plexus and represents an ultrafiltrate of plasma. Therefore, the total protein content is very low compared with plasma. However, the composition of CSF is modified by the choroid plexus, where Dkk-3 could be transferred from the plasma by an active transport mechanism, or produced locally by the epithelial lining of the plexus. Our data demonstrate that these epithelial cells of the choroid plexus produce Dkk-3 and therefore it is likely, that at least a fraction of Dkk-3 present in CSF is derived from this source.
Furthermore, DKK3 gene expression has been reported in the human cortex especially in pyramidal cells (Ftouh et al. 2005) and our data demonstrate that these cells also produce Dkk-3 protein. Diffusion of the protein through the brain tissue might also contribute to Dkk-3 CSF levels.
Dickkopf homolog-3 as a diagnostic biomarker for dementia
β-amyloid (1–42), one of the best established and widely used biomarkers for diagnosis of AD (Fagan et al. 2009; Shaw et al. 2009; Tapiola et al. 2009), segregates the studied cohorts with high sensitivity and specificity. Given the increased Dkk-3 and decreased β-amyloid (1–42) levels in CSF of AD patients, the ratio of β-amyloid (1–42)/Dkk-3 was analyzed as a classifier for disease by ROC analysis. While the accuracy to discriminate between AD patients and controls did not change significantly [because of the already excellent accuracy when using β-amyloid (1–42) levels alone], the sensitivity and specificity of the ratio as classifier to segregate controls from MCI and MCI from AD patients was clearly superior to β-amyloid (1–42) levels, indicating the value of Dkk-3 as an additional biomarker.
However, it is well established that the measurement of β-amyloid (1–42), tau, and phospho-tau-181 in CSF can be used to diagnose AD with high sensitivity and specificity, and the additional information provided by Dkk-3 levels might not justify its use for routine diagnosis in CSF. Alternatively, the research for plasma-derived biomarkers is of high importance, because the invasive lumbar puncture and collection of CSF limits the diagnosis of dementia. We observed an increase of Dkk-3 levels associated with AD in plasma similar to that in CSF, indicating that the increase in plasma levels might be directly associated with disease status and that Dkk-3 levels in CSF and plasma are interrelated either by active or passive transport over the blood–brain barrier. Thus, the measurement of Dkk-3 in plasma may help to overcome this problem and may be useful in diagnosing AD. ROC analysis of Dkk-3 plasma levels as a classifier for AD diagnosis revealed a fair accuracy, suggesting that Dkk-3 plasma levels indeed can be useful for the diagnosis of dementia when weighed in combination with other molecular markers.
Conclusions
In summary, this study revealed the presence of high levels of Dkk-3 in CSF which is at least in part secreted by epithelial cells of the choroid plexus. With a recently established sensitive and specific IEMA for Dkk-3 significant changes in the plasma and CSF levels were revealed in patients suffering from AD, while Dkk-3 levels in samples derived from depression or MCI patients were unchanged compared with control subjects. Future work will be set up to study the potential role of Dkk-3 in the development of AD and to further analyze its utility as a diagnostic marker for neurodegenerative diseases.
Acknowledgement
The authors wish to thank Roswitha Plank for her excellent technical support.
Abbreviations used
- AD
Alzheimer’s disease
- AUC
area under the ROC curve
- BSA
bovine serum albumin
- Dkk
Dickkopf homolog
- IEMA
immunoenzymometric assay
- mAb
monoclonal antibody
- MCI
mild cognitive impairment
- MS
mass spectrometry
- PBS
sodium phosphate buffer
- recDkk-3
recombinant human Dkk-3
- ROC
receiver operating characteristics
Footnotes
Disclosure statement
There are no actual or potential conflicts of interest.
References
- Bian H, Van Swieten JC, Leight S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70:1827–1835. doi: 10.1212/01.wnl.0000311445.21321.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibl M, Mollenhauer B, Esselmann H, et al. Cerebrospinal fluid neurochemical phenotypes in vascular dementias: original data and mini-review. Dement. Geriatr. Cogn. Disord. 2008;25:256–265. doi: 10.1159/000115975. [DOI] [PubMed] [Google Scholar]
- Blasko I, Lederer W, Oberbauer H, Walch T, Kemmler G, Hinterhuber H, Marksteiner J, Humpel C. Measurement of thirteen biological markers in CSF of patients with Alzheimer’s disease and other dementias. Dement. Geriatr. Cogn. Disord. 2006;21:9–15. doi: 10.1159/000089137. [DOI] [PubMed] [Google Scholar]
- Blennow K. CSF biomarkers for mild cognitive impairment. J. Intern. Med. 2004;256:224–234. doi: 10.1111/j.1365-2796.2004.01368.x. [DOI] [PubMed] [Google Scholar]
- Blennow K. CSF biomarkers for Alzheimer’s disease: use in early diagnosis and evaluation of drug treatment. Expert Rev. Mol. Diagn. 2005;5:661–672. doi: 10.1586/14737159.5.5.661. [DOI] [PubMed] [Google Scholar]
- Desai AK, Grossberg GT. Diagnosis and treatment of Alzheimer’s disease. Neurology. 2005;64:S34–S39. doi: 10.1212/wnl.64.12_suppl_3.s34. [DOI] [PubMed] [Google Scholar]
- Diep DB, Hoen N, Backman M, Machon O, Krauss S. Characterisation of the Wnt antagonists and their response to conditionally activated Wnt signalling in the developing mouse forebrain. Brain Res. Dev. Brain Res. 2004;153:261–270. doi: 10.1016/j.devbrainres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann. Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradinger EA, Bitan G. En route to early diagnosis of Alzheimer’s disease – are we there yet? Trends Biotechnol. 2005;23:531–533. doi: 10.1016/j.tibtech.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Ftouh S, Akbar MT, Hirsch SR, de Belleroche JS. Down-regulation of Dickkopf 3, a regulator of the Wnt signalling pathway, in elderly schizophrenic subjects. J. Neurochem. 2005;94:520–530. doi: 10.1111/j.1471-4159.2005.03239.x. [DOI] [PubMed] [Google Scholar]
- Goodwin AM, Sullivan KM, D’Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev. Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- Henley SM, Bates GP, Tabrizi SJ. Biomarkers for neurodegenerative diseases. Curr. Opin. Neurol. 2005;18:698–705. doi: 10.1097/01.wco.0000186842.51129.cb. [DOI] [PubMed] [Google Scholar]
- Hermann M, Pirkebner D, Draxl A, Berger P, Untergasser G, Margreiter R, Hengster P. Dickkopf-3 is expressed in a subset of adult human pancreatic beta cells. Histochem. Cell Biol. 2007;127:513–521. doi: 10.1007/s00418-007-0278-6. [DOI] [PubMed] [Google Scholar]
- Humpel C, Marksteiner J. Peripheral biomarkers in dementia and Alzheimers disease. In: Ritsner MS, editor. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes, Volume III: Metabolic and Peripheral Biomarkers. Springer; Netherlands, Dordrecht: 2009. pp. 3–12. [Google Scholar]
- Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch. Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- Krupnik VE, Sharp JD, Jiang C, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- Kupatt C, Horstkotte J, Vlastos GA, et al. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Wiltfang J. Neurochemical dementia diagnostics: state of the art and research perspectives. Proteomics. 2008;8:1292–1301. doi: 10.1002/pmic.200700703. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- McKeel DW, Jr, Price JL, Miller JP, Grant EA, Xiong C, Berg L, Morris JC. Neuropathologic criteria for diagnosing Alzheimer disease in persons with pure dementia of Alzheimer type. J. Neuropathol. Exp. Neurol. 2004;63:1028–1037. doi: 10.1093/jnen/63.10.1028. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Bibl M, Wiltfang J, Steinacker P, Ciesielczyk B, Neubert K, Trenkwalder C, Otto M. Total tau protein, phosphorylated tau (181p) protein, beta-amyloid(1–42), and beta-amyloid(1–40) in cerebrospinal fluid of patients with dementia with Lewy bodies. Clin. Chem. Lab. Med. 2006;44:192–195. doi: 10.1515/CCLM.2006.035. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Ray S, Britschgi M, Herbert C, et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat. Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, Pirttila T. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch. Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- Untergasser G, Steurer M, Zimmermann M, Hermann M, Kern J, Amberger A, Gastl G, Gunsilius E. The Dickkopf-homolog 3 is expressed in tumor endothelial cells and supports capillary formation. Int. J. Cancer. 2008;122:1539–1547. doi: 10.1002/ijc.23255. [DOI] [PubMed] [Google Scholar]
- Wu W, Glinka A, Delius H, Niehrs C. Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/beta-catenin signalling. Curr. Biol. 2000;10:1611–1614. doi: 10.1016/s0960-9822(00)00868-x. [DOI] [PubMed] [Google Scholar]
- Zenzmaier C, Sklepos L, Berger P. Increase of Dkk-3 blood plasma levels in the elderly. Exp. Gerontol. 2008a;43:867–870. doi: 10.1016/j.exger.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Zenzmaier C, Untergasser G, Hermann M, Dirnhofer S, Sampson N, Berger P. Dysregulation of Dkk-3 expression in benign and malignant prostatic tissue. Prostate. 2008b;68:540–547. doi: 10.1002/pros.20711. [DOI] [PubMed] [Google Scholar]