Abstract
Objective
This study tested whether global and regional brain volumes correlated with body mass index (BMI) and increases in BMI over 1-year follow-up.
Methods
Eighty-three young females (M age = 18.4, SD = 2.8; BMI range = 17.3–38.9) were scanned using magnetic resonance imaging (MRI). Voxel-based morphometry was used to assess global brain volume and regional grey matter (GM) and white matter (WM) volumes in regions implicated in taste, reward, and inhibitory control.
Results
Obese participants had less total GM volume than lean and overweight participants. Obese participants had lower total WM volume than overweight participants. BMI correlated with higher WM volumes in the middle temporal gyrus, fusiform gyrus, parahippocampal gyrus, Rolandic operculum, and dorsal striatum. Trend-level reduced GM volumes in the superior frontal gyrus and middle frontal gyrus were related to increases in BMI over 1-year follow-up.
Conclusion
Findings suggest that BMI is related to global and regional differences in brain matter volume in female adolescents. Most importantly, findings suggest that low GM volume in regions implicated in inhibitory control are related to future weight gain. Results taken in conjunction with prior findings suggest that abnormalities in regional GM volumes, but not WM volumes increase the risk for future weight gain and abnormalities in regional WM volumes but not GM volumes are secondary to weight gain.
Keywords: obesity, weight gain, voxel-based morphometry, brain volume, impulsivity
Introduction
In the US 65% of adults are overweight or obese (1) and obesity rates in children and adolescents have increased dramatically over the past decennia (2). Obesity is associated with increased risk for mortality, atherosclerotic cerebrovascular disease, coronary heart disease, colorectal cancer, hyperlipidemia, hypertension, gallbladder disease, and diabetes mellitus, resulting in over 111,000 deaths annually in the US (3). Obesity is also associated with poor cognitive function and neuro-degenerative disorders, such as dementia (4,5).
There is evidence that obesity is associated with anomalous regional metabolism and neural activity, particularly in areas of the brain involved in reward and taste. Functional magnetic resonance imaging (fMRI) studies have found that obese versus lean humans show greater responsivity in gustatory regions (insula/frontal operculum), somatosensory regions (e.g., Rolandic operculum), and reward processing regions (e.g., caudate, putamen, amygdala, orbitofrontal cortex [OFC]) in response to palatable food cues (6–9). Further, there is limited evidence that this hyper-responsivity increases risk for future weight gain (10). Lean adolescents at high- versus low-risk for obesity show greater responsivity in the dorsal striatum and parietal/frontal operculum during palatable food receipt (11). These findings collectively suggest that elevated responsivity of reward and somatosensory regions increase risk for obesity.
Yet, obese versus lean humans have fewer dopamine (DA) receptors in striatal regions (12,13) and show reduced striatal response to palatable food intake (14,7). Further, blunted dorsal striatal response to chocolate milkshake receipt also predicts future increases in BMI for young women with the A1 allele of the single nucleotide polymorphism referred to as the TaqIA (15), which is associated with lower D2 striatal receptor availability and reduced striatal resting metabolism (16,17). Interestingly, blunted response of the dorsal striatum and frontal operculum to images of palatable foods also predicts future increases in BMI for those with a TaqIA A1 allele (8).
One explanation for these seemingly inconsistent findings is that some of these effects reflect initial vulnerability factors, whereas others result from obesity. Firing of DA neurons in reward regions shifts from food intake to cues that predict food intake after conditioning in rats (18,19), implying that overeating is related to increased responsivity of regions that encode the reward value of food images and cues. Further, overeating leads to reduced D2 receptor density, D2 sensitivity, and reward sensitivity in rats (20–22) and reduced striatal response to food in humans (23), suggesting that overeating leads to receptor down-regulation in dopamine-based reward regions.
There is also evidence that obesity is related to structural/volumetric brain differences. BMI has correlated with low global brain volume (24,25) as well as with regional grey matter (GM) and white matter (WM) volume differences (e.g, 26). The function of GM, which consists of neuronal cell bodies, neuropil, glial cells and capillaries, is to route sensory or motor stimuli to interneurons of the central nervous system to create a response to the stimuli through chemical synapse activity (27). WM contains myelinated axon tracts that connect different areas of GM in the brain. Myelin acts as an insulator increasing the speed of transmission of all nerve signals (27). Pannacciulli et al. (26) has found that obese versus lean individuals have significantly lower GM volume in regions implicated in taste processing (postcentral gyrus and frontal operculum/extended insula) and reward (putamen). These regional volumes were negatively correlated with fasting plasma leptin concentration, a hormone that is produced in proportion to the amount of body fat (28). Obese versus lean showed high GM volume in the middle occipital gyrus, inferior frontal gyrus, and cuneus and higher WM volume in the striatum (26). Taki et al. (25) has found negative correlations between BMI and GM volume in the fusiform gyrus, superior parietal lobe, precentral gyrus, inferior- and superior frontal gyri, midbrain, uncus, cerebellum, and precuneus as well as positive correlations between BMI and GM volume in the posterior lobe of cerebellum, temporal gyrus, thalamus, cingulate gyrus, and caudate. Walther et al. (29) has found negative correlations between BMI and GM volumes in the OFC, inferior- and middle frontal gyri, parahippocampal gyrus, lingual gyrus, and cerebellum. Additionally, Horstmann et al. (30) has found a negative correlation between BMI and GM volume in the dorsolateral prefrontal cortex, but a positive correlation between BMI and GM volume in the OFC, putamen, and hypothalamus. Maayan et al. (31) has found that obese versus lean adolescents have significantly lower GM volume in the OFC. Although Haltia and colleagues (32) did not find significant differences in GM volume, WM volume was greater in the temporal gyrus, fusiform gyrus, parahippocampal gyrus, brain stem and cerebellum in obese versus lean individuals. Finally, Raji et al. (33) has found negative correlations between BMI and GM and WM volume in the OFC, ACC, medial temporal lobe, hippocampus, putamen, globus pallidus, and thalamus.
The abovementioned cross-sectional data provide evidence of a complex pattern of volumetric differences in obesity. Most studies show that obesity is associated with reduced GM volume in regions involved in taste (postcentral gyrus, frontal operculum/insula) and inhibitory control (inferior-, middle-, and superior frontal gyri). Findings with regard to GM volume in regions involved in reward (OFC, caudate, putamen, midbrain) are somewhat mixed. The few studies that examined the association between BMI and regional WM volume have also provided inconsistent results. Further, it is unclear whether structural/volumetric brain differences reflect initial vulnerability factors or are the result of obesity. One hypothesis is that an increase in BMI results in volumetric changes in the brain. For example, inflammatory cytokines/adiopokines such as fibrinogen, IL-1β, IL-6, and C-reactive protein are associated with excess adipose tissue (e.g., 34,35) and elevated levels of such inflammatory markers are positively correlated with insulin resistance, metabolic syndrome, and type 2 diabetes (e.g., 36,37). IL-6 levels are negatively correlated with global brain volume and regional GM volume in the hippocampus and medial PFC (38). In fact, IL-6 levels mediate the association between body fat and hippocampal grey matter volume (39). Further, monkeys on a long-term, calorie-restricted diet show reduced levels of IL-6 and decreased IL-6-related global GM and WM atrophy, as well as GM atrophy in parietal and temporal regions (40). Both rat and human studies have shown that a low-calorie diet restricts protein expression of IL-6 (e.g., 41). In sum, inflammatory markers may be mechanisms by which obesity is related to low GM volume in the brain. Moreover, Haltia et al. (32) has hypothesized that an increase in body fat may enhance the density of myelin, which increases WM volume. This is supported by their findings that obese versus lean individuals show significantly higher concentrations of serum free acids and that serum free fatty acid concentration and regional WM volume in obese subjects were positively associated. This is also supported by rodent studies indicating that the hypothalamic metabolism of fatty acids can modify feeding behavior (42). Haltia et al. (32) also found that global WM as well as regional WM volumes in the temporal lobe were partially reduced by a controlled very low-calorie diet for 6 weeks in obese subjects. Changes in global or regional gray matter were non-significant (32). Animal studies have also found that overeating is related to cognitive decline while caloric restriction slows brain aging (43). These findings suggest that WM brain volumetric changes could, at least partly, be secondary to weight change.
An alternative hypothesis is that low GM volume and/or high WM volume increases risk for obesity. Comparable to the reward deficit model of obesity (44), it is possible that low GM and/or high WM volume in reward regions attenuates reward from food, prompting increased intake in a compensatory fashion. It has also been theorized that impulsive individuals are more sensitive to cues for reward and more vulnerable to the omnipresent temptation of appetizing foods (45,46), increasing risk for unhealthy weight gain. Trait impulsivity is thought to result in greater sensitivity to reward-predictive cues, which may contribute to compulsive food-seeking behavior (47). Thus, it is possible that low GM and/or high WM volume in regions involved in inhibitory control increases the likelihood of giving into tempting palatable foods. Both impulsivity and obesity have found to be related to less GM volume in the OFC (31,48). Obese versus lean adults also show less GM volume in the prefrontal cortex (26), a region that modulates inhibitory control. Obese versus lean young women show less activation of prefrontal regions (e.g., middle frontal gyrus, ventrolateral prefrontal cortex) when trying to inhibit responses to unhealthy food images and show behavioral evidence of reduced inhibitory control (49). Relative to obese controls, adults who achieve lasting weight loss show greater activation of inhibitory regions (superior frontal- and middle temporal gyri) in response to palatable food images (50). Reduced activation in the dorsolateral prefrontal cortex in response to palatable food images also predicts increased ad lib food intake over the next 3 days in men and women (51). To our knowledge, no other study has tested whether reduced GM volume and/or increased WM volume is related to future weight gain.
We think it is important to use voxel-based morphometry to test whether volumetric differences in certain brain regions are related to future BMI as this may provide us with a better understanding of the risk processes that give rise to obesity. We hypothesized that reduced GM and increased WM in regions involved in taste (anterior insula/frontal operculum, Rolandic operculum), reward (OFC, caudate, putamen), and inhibitory control (inferior-, middle-, and superior frontal gyri) would predict future increases in BMI.
In an attempt to replicate previous findings, we also tested the correlation of BMI to global and regional brain matter volumes. Based on the abovementioned studies, we hypothesized that BMI is correlated with reduced overall GM volume and with reduced GM volume in brain regions involved in taste, reward, and inhibitory control and increased WM volume in the caudate, putamen, inferior-, middle- and superior temporal gyri, and parahippocampal gyrus.
Methods
Participants
Participants were 83 young women (M age = 18.4; SD = 2.8; 6.0% African Americans, 78.3% European Americans, 4.8% Native Americans, 1.2% Native Hawaiian or other Pacific Islander, and 9.6% mixed racial heritage). Thirty-eight subjects (M age = 15.7, SD = .94; M BMI =24.3; SD = 4.98; BMI range = 17.3–38.9) were recruited from a larger prevention trial of female high school students with body image concerns. Individuals in the larger study who gave consent to be contacted about other studies were asked to participate in a side-study on the neural response to presentation of food. Another 45 subjects (M age = 20.7, SD = 1.5; M BMI =27.9; SD = 2.6; BMI range = 20.7–33.2) were drawn from a study evaluating the efficacy of a behavioral weight loss treatment using fMRI. Participants in both samples were scanned at baseline prior to the trials. Exclusion criteria were current Axis I psychiatric disorder (including eating disorders), any use of psychoactive drugs, and standard MRI contra-indications. Participants and their parents (if minor) provided written informed consent. The protocol was approved by the local institutional review board.
Measures
Body mass
Body mass index (BMI = kg/m2) was used to reflect adiposity (52). After removal of shoes and coats, height was measured to the nearest millimeter using a stadiometer and weight was assessed to the nearest 0.1 kg using a digital scale. Two measures of each were obtained and averaged. BMI correlates with direct measures of total body fat such as dual energy x-ray absorptiometry (r = .82 to .87; 53,54) and with health measures such as blood pressure (52). Participants provided BMI data at baseline and at 6- and 12-month follow-ups. Participants aged 20 years or younger were categorized as lean, overweight, or obese based on the Centers for Disease Control BMI-for-age growth chart for girls (55). For those aged 21 years and older (N=29), participants were categorized based on adult cut-offs.
MRI acquisition
Scanning was performed in a Siemens Allegra 3-Tesla, head-only MRI scanner. High-resolution structural MRI scans (160 sagittal slices, 1×1×1 mm, FOV: 256×256 mm2, TR = 2000 ms, TE = 30 ms, flip angle = 80°) were acquired using inversion recovery T1-weighted sequence (MP-RAGE) along the AC-PC transverse, oblique plane as determined by the midsagittal section.
Non-brain tissue was removed using the Brain Extraction Tool (BET; 56) in FSL (Analysis Group, FMRIB, Oxford, UK). Data were manually realigned to the AC-PC and analyzed using SPM8 software (Wellcome Department of Imaging Neurosicence, London, UK) in MATLAB (Mathworks, Inc., Sherborn, MA; 57). T1 images were preprocessed using the VBM8 Toolbox developed by Christian Gaser (University of Jena, Psychiatry Department) in SPM8. Images were normalized to MNI space using high-dimensional Dartel normalization segmented into GM, WM, and cerebrospinal fluid (CSF). Modulated data were used in analyses. Images were then smoothed to an 8 mm full-width at half maximum (FWHM) Gaussian kernel.
Statistical analysis
All analyses were done using SPM8 software. Participants were categorized as obese, overweight, and lean based on their BMI to test global brain volume differences among these three groups. Between-group comparisons were performed to test whether obese and overweight individuals show lower global GM volume and higher global WM volume compared to lean individuals. Group differences in global GM and WM volumes were tested by means of the following comparisons: 1) obese (N = 17) versus lean (N = 31), 2) overweight (N = 36) versus lean, 3) obese versus overweight. To test the hypothesis that BMI correlates with lower regional GM volumes and higher regional WM volumes, multiple regression analyses (N = 83) were performed with BMI as independent variable and regional GM/WM volumes as dependent variables. Total GM volume was controlled for in all GM analyses and total WM volume was controlled for in all WM analyses. To test the hypothesis that GM/WM volumes are related to change in BMI over 1-year follow-up, multiple regression analyses were performed (N = 81). Change in BMI was defined as the slope calculated from the data points at baseline, 6-month follow-up, and 12-month follow-up. Change in BMI and initial BMI (control variable) were entered as covariates. Again, total GM volume was controlled for in all GM analyses and total WM volume was controlled for in all WM analyses.
Region of interest (ROI) masks were created using the WFUPickatlas (58) to test the specific hypotheses. For hypotheses involving GM volumes, ROIs included the insula, postcentral gyrus, caudate, putamen, and inferior-, middle-, and superior frontal gyri. For hypotheses involving WM volumes, ROIs included the caudate, putamen, inferior-, middle-, superior temporal gyri, and parahippocampal gyrus. T-maps were thresholded at p<0.001 uncorrected with a cluster threshold of 3. Predicted activations were considered to be significant at p<.0.05 after correcting for multiple comparisons (pFDR) across the total number of voxels across all ROIs (58). Peaks outside the hypothesized regions were considered to be significant at p<0.05 FDR corrected across the whole brain. We derived effect sizes (r) from the z values (z/√N)
Results
Group differences in global grey matter and white matter volume
There was a significant difference in global GM volume among the three groups, F(2)=5.5, p=.006. Post hoc tests showed that obese participants (M=499.54, SD=49.68; p=.011) had less total GM volume than lean participants (M=542.78, SD=60.30). Obese participants also had less total GM volume than overweight participants (M=542.90, SD=40.52) (p =.010). There was no difference between overweight and lean participants in total GM volume.
There was a significant difference in overall WM volume among the three groups, F(2)=3.80, p=.027. Obese participants (M=452.27, SD=45.06) had lower total WM volume than overweight participants (M=486.34, SD=43.84) (p=.025). There was no difference in WM volume between obese and lean participants (M=465.81, SD=46.04) or between overweight and lean participants.
Correlations between BMI and grey matter volume in a priori ROIs
There were no significant correlations between BMI and GM volume in the a priori ROIs. Correlation analyses did reveal a positive correlation between BMI and GM volume in the right middle occipital gyrus (Table 1), which was outside the hypothesized regions.
Table 1.
Locations of significant region differences in grey matter and white matter volume
| Region and regression condition |
L/R | xa | y | z | Vb | Z value |
pFDR corrected d |
r (Z/√N) |
|---|---|---|---|---|---|---|---|---|
| Positive correlation with BMI | ||||||||
| Grey matter | ||||||||
| Middle occipital gyrus | R | 44 | −90 | 1 | 833 | 5.40 | 0.004c | 0.59 |
| White matter | ||||||||
| Middle occipital gyrus | R | 29 | −84 | 9 | 1868 | 4.87 | 0.03c | 0.53 |
| Middle temporal gyrus | R | 41 | −63 | 6 | 141 | 4.11 | 0.04 | 0.45 |
| L | −30 | −72 | 16 | 56 | 3.72 | 0.04 | 0.41 | |
| R | 45 | −52 | −2 | 20 | 3.50 | 0.04 | 0.38 | |
| L | −50 | −45 | −2 | 57 | 3.38 | 0.04 | 0.37 | |
| Fusiform gyrus | L | −30 | −73 | −18 | 251 | 4.37 | 0.01 | 0.48 |
| Parahippocampal gyrus | L | −29 | −60 | −8 | 65 | 4.00 | 0.02 | 0.44 |
| R | 14 | −45 | 3 | 257 | 3.91 | 0.02 | 0.43 | |
| L | −26 | −25 | −11 | 70 | 3.45 | 0.02 | 0.38 | |
| R | 29 | −28 | −9 | 4 | 3.13 | 0.02 | 0.34 | |
| Rolandic operculum | L | −53 | −4 | 10 | 193 | 3.76 | 0.02 | 0.41 |
| Putamen | R | 20 | 6 | 6 | 8 | 3.20 | 0.05 | 0.35 |
| Caudate | R | 18 | 15 | 3 | 6 | 3.10 | 0.04 | 0.34 |
| Negative correlation with future increases in BMI | ||||||||
| Grey matter | ||||||||
| Superior frontal gyrus | R | 8 | −4 | 72 | 545 | 3.99 | 0.06 | 0.44 |
| L | −29 | 8 | 66 | 249 | 3.57 | 0.06 | 0.39 | |
| Middle frontal gyrus | L | −48 | 9 | 51 | 90 | 3.66 | 0.06 | 0.40 |
Stereotactic coordinates in MNI space (Internet: http://mni.mcgill.ca/). Coordinates of the voxel of greatest activation within the MNI coordinate system are displayed.
Spatial extent (in contiguous voxels)
FDR corrected <0.05 across the whole brain
Correlations between BMI and white matter volume in a priori ROIs
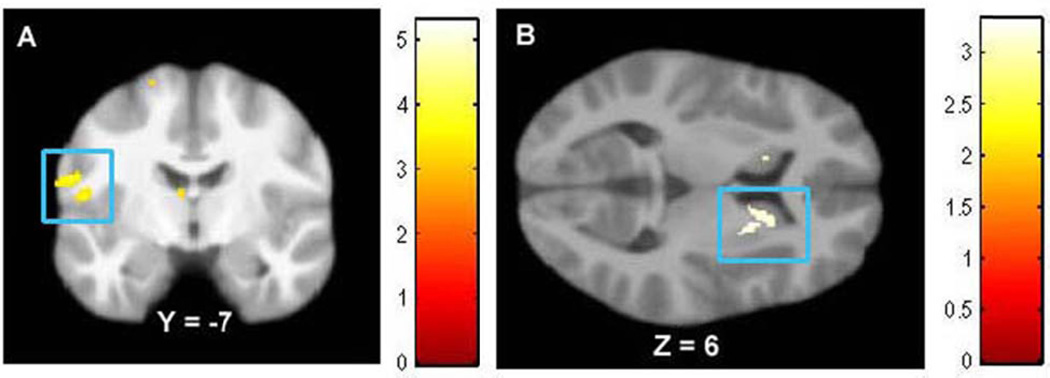
BMI correlated positively with WM volume in the following a priori ROIs (Table 1): bilateral middle temporal gyrus, left fusiform gyrus, bilateral parahippocampal gyrus, left Rolandic operculum (Figure 1A), and right dorsal striatum (Figure 1B). BMI also correlated positively with WM volume outside the a priori ROIs, namely in the left middle occipital gyrus (Table 1). Effect sizes were all medium to large per Cohen’s criteria (59)
Figure 1.
BMI correlated with greater WM volume in A) Rolandic operculum (MNI coordinate: −59, −7, 19, Z = 3.54, pFDR = 0.02), and B) dorsal striatum (MNI coordinates: 20, 6, 6, Z = 3.20, pFDR = 0.05; 18, 15, 3, Z = 3.10, pFDR = 0.04).
Relation between grey- and white matter volume and change in BMI from baseline to 1-year follow-up
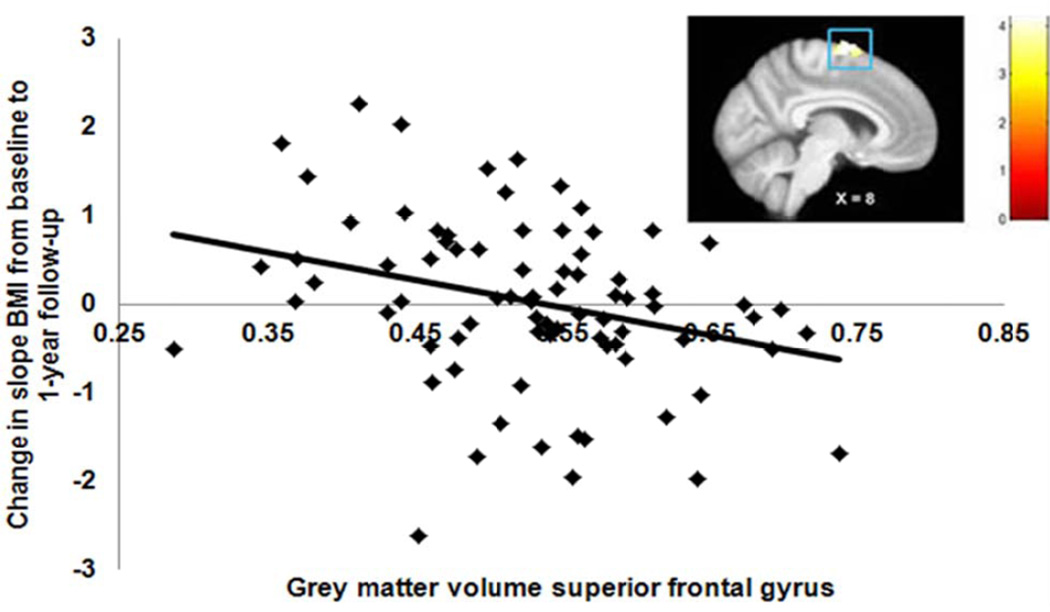
The average change in slope BMI across the total sample over the 1-year follow-up period was -.4 (SD = 0.95 range = −6.0 to 2.6). Trend-level reduced GM volumes (pFDR = 0.06) in regions involved in inhibitory control, namely the bilateral superior frontal gyrus and left middle frontal gyrus, were related to increases in BMI over 1-year follow-up (Figure 2; Table 1), while controlling for baseline BMI. WM volume was not correlated with change in BMI. Effect sizes were medium per Cohen’s criteria (59).
Figure 2.
Reduced GM in the superior frontal gyrus (MNI coordinate: 8, −4, 72, Z = 3.99, pFDR = 0.06) is related to increases in BMI from baseline to 1-year follow-up, while controlling for initial BMI.
Discussion
In the current study, obese female adolescents show significantly lower overall GM volume compared to overweight and lean female adolescents, suggesting greater global brain atrophy. This result converges with findings of previous studies in middle-aged obese adults (24,25). There was no difference between overweight and lean individuals in global GM volume suggesting that the relation between BMI and global GM volume is non-linear. A possible explanation could be that obese individuals compared to individuals with a lower weight experience many more biological changes (e.g., hypertension, insulin resistance) that negatively affect global GM volume.
Although obese individuals had lower overall GM volume compared to lean and overweight individuals, we did not find significant negative associations between BMI and regional GM volumes. This result is in contrast with the findings of two previous studies (26,29), but comparable with those from another (32). A possible explanation for these inconsistent findings is that participants in both the current study and in Haltia et al. (32) were overall younger and less obese compared to those in the other two studies. It is possible that only more severe and chronic obesity negatively influences regional GM volume, in line with the thesis that volumetric changes are at least in part secondary to elevated adipose tissue levels. Further, Taki and colleagues (25) found a significant correlation between BMI and reduced regional GM in men, but not women, suggesting possible sex differences in the relation between BMI and regional GM volume. Taki et al. (25) posit that the null findings in women may be the result of gender differences in fat distribution, with visceral fat predominating in men and subcutaneous fat predominating in women (60). Visceral fat is likely indicative of metabolic syndrome (61,62), which is associated with elevated serum levels of inflammatory markers. As discussed earlier, inflammatory markers have been associated with changes in GM and WM volume (38,39). However, Walther et al. (29) did find significant correlations between BMI and GM volume in women, suggesting that other sample or study characteristics may play a role in the difference in findings. Additional studies are needed to ascertain whether severity of obesity, gender and types of fat distribution affect regional GM volume differently.
Interestingly, we found a positive correlation between BMI and regional GM volume in the right middle occipital gyrus. This result was not an a priori defined region of interest, but is comparable with the findings of a previous study (26), in which GM density in the middle occipital gyrus was greater in obese compared to lean individuals. Occipital regions are typically involved in visual processing such as object recognition, color perception, and selective attention (63,64). Using a food-based visual attention task, one neuroimaging study has found that BMI positively correlates with selective attention to appetizing food and greater activation in reward processing regions including the anterior insula, ventrolateral PFC and lateral OFC (10). Further, a meta-analysis of visual processing of food and non-food cues found that exposure to food images resulted in elevated activation in the lateral of GM complex (a region extending from the posterior fusiform gyrus to the inferior occipital gyrus) (65). Given that individuals with a higher BMI show increased selective attention toward appetitive stimuli, it is possible that greater GM in the visual cortex (e.g., occipital region) reflects this difference in neural activity. However, it is important to note that increased GM volume measured with VBM can also result from several factors that we were unable to test, including differences at the cellular level and different folding patterns (66,67). Therefore, it will be important for future studies to define which of these possibilities underlies the observed positive relation.
Comparable to a previous study (32), we did not find significant differences in global WM volume between obese and lean individuals or between overweight and lean individuals. However, considering that we did find greater overall WM in overweight compared to obese individuals, it is also possible that our null findings can be explained by a small sample size. Only 17 participants in our sample were obese, which potentially limited our statistical power to detect small effects. It will important for future studies to explore the global WM volume differences in larger samples sizes.
BMI correlated positively with WM volumes in bilateral middle temporal gyrus, left fusiform, and bilateral parahippocampal gyrus, left Rolandic operculum, and right dorsal striatum (putamen and caudate), all regions previously found to be involved in palatable food receipt, food cues, and reward (68–72). Further, we also found a positive correlation between BMI and WM volume in the middle occipital gyrus. Overall, these results converge with previous findings (32,26). Moreover, Haltia et al. (32) found that after obese participants went on a low calorie diet for 6 weeks, global WM volume as well as regional WM volume in the left temporal lobe was reduced. Haltia et al. (32) hypothesized that fatty acid excess in obesity could result in pathological lipid metabolism in the brain, and this may increase brain WM volume. It is therefore possible that WM expansion is secondary to fat accumulation in obesity. Although we found positive relations between BMI and regional WM volumes, it is important to consider that it is unknown whether the higher WM volume actually reflects increased myelinization. Previous studies have found that higher BMI is associated with regional WM damage (73,74). Further, obese mice appear to have a lower amount of myelin compared to normal mice (75). Diffusion tensor imaging (DTI) is a neuroimaging technique able to examine the microstructural integrity of white matter. Increased myelinization could be indicated by a higher fractional anisotropy value while white matter damage by a lower one. Further studies determining whether the higher volume of white matter observed in obese individuals actually reflects increased myelinization are warranted.
Trend-level reduced regional GM volumes in the prefrontal cortex were associated with an increase in slope BMI from baseline to 1-year follow-up, controlling for initial BMI. Although studies have explored the associations between BMI and brain volume (e.g., 26,25) and the effects of dieting on regional GM/WM volumes in obese subjects (32), this is the first prospective study to examine the relation between regional GM/WM volumes and future weight gain. These findings dovetail with evidence that obese versus lean adults show less GM volume in the prefrontal cortex (26), less activation of prefrontal regions (e.g., middle frontal gyrus, ventrolateral prefrontal cortex) when trying to inhibit responses to unhealthy food images, and show behavioral evidence of reduced inhibitory control (49). The prefrontal cortex has been commonly implicated in the inhibition of inappropriate responses, control of goal-directed behaviors, and ability of error detection/correction (76) and could have a role in decisions to terminate feeding (77) and whether or not to eat unhealthy food. Collectively, these data may suggest that reduced GM volume in regions involved in inhibitory control may increase the risk for giving into tempting high-fat/sugar foods, resulting in overeating and weight gain.
In contrast to our hypothesis, we did not find a significant relation between WM volume and future increases in BMI. Haltia and colleagues (32) found that after following a very-low-calorie-diet over a period of 6 weeks, obese individuals showed a significant reduction in global and regional WM volume in the temporal lobe while changes in global and regional GM were non-significant. It is therefore possible that abnormalities in regional GM volumes, but not WM volumes increase risk for future weight gain and abnormalities in regional WM volumes but not GM volumes are secondary to weight gain. An alternative possibility is that there are reciprocal relations between BMI and regional GM/WM volumes. For example, reduced GM in the prefrontal cortex may lead to less inhibitory control towards eating high-fat/sugar foods, which may lead to overeating and weight gain, and consequently may lead to higher regional WM due to fat accumulation. Unfortunately, we were not able to test the effect of change in BMI on brain volume, as we did not have repeat scans for these subjects at 1-year follow-up. Either possibility suggests that examining bi-directional relations between BMI and regional brain volume in prospective repeated-measure studies may provide important information above and beyond the current findings.
It is important to consider the limitations of this study. First, due to possible registration errors and smoothing, it cannot be excluded that some GM signal is included in the total WM signal and vice versa. Second, the current study was conducted solely with young females, thus results should be generalized with caution to males and to adults. Third, due to our relatively small sample size, the current study was sufficient to detect large and medium effects, but was not adequate to detect small effects. Fourth, although BMI is widely used to assess excess adiposity, is inexpensive, and shows high test-retest reliability, it does not distinguish between increased mass in the form of fat, lean tissue, or bone and hence can lead to significant misclassification (78,79). However, it is important to note that BMI correlates highly with direct measures of total body fat such as dual energy x-ray absorptiometry in female (r = 0.85 – 0.87) and male adolescents (r = 0.82 – 0.89) (53,54). Nonetheless, it is possible that by using a more accurate metric of access fat mass (e.g., dual energy x-ray absorptiometry), additional or stronger correlations with volumetric brain differences would have been found. Future studies testing the correlations between fat mass and volumetric brain differences are needed to examine these relations more closely.
Despite the aforementioned limitations, the current findings suggest that elevated weight is associated with low global GM volume and high WM volumes in regions previously found to be involved in palatable food receipt, food cues, and reward. The current findings also suggest that reduced GM volumes in regions related to inhibitory control are associated with future increases in BMI. Undoubtedly, the relations between BMI and GM/WM volumes are complex and further consideration of other variables (e.g., medical conditions, genetics) is warranted. However, while the specific mechanisms underlying these volumetric differences remain to be investigated, our findings have important public health implications because they suggest that regional and global brain volume abnormalities are related to BMI and increases in BMI at a relatively young age, potentially resulting in greater risk for future declines in cognition or other brain functions.
Acknowledgments
This research was supported by a Roadmap Supplement for Interdisciplinary Research in Behavioral and Biological Sciences (R1MH64560A).
References
- 1.Hedley AA, Odgen CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2000. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 4.Jeong SK, Nam HS, Son MH, Son EJ, Cho KH. Interactive effect of obesity indexes on cognition. Demet Geriatr Cogn Disord. 2005;19:91–96. doi: 10.1159/000082659. [DOI] [PubMed] [Google Scholar]
- 5.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Stice E, Spoor S, Bohon C, Veldhuizen M, Small DM. Relation of reward from food intake and anticipated intake to obesity: A functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity. doi: 10.1038/oby.2011.168. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31:4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 14.Geha P, et al. Smokers have differential brain response to food in regions that predicted weight gain in non-smokers. PloS One. 2011 in press. [Google Scholar]
- 15.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble EP, Gottschalk LA, Fallon JH, Ritchie TL, Wu JC. D2 dopamine receptor polymorphism and brain regional glucose metabolism. Am J Med Genet. 1997;74:162–166. [PubMed] [Google Scholar]
- 17.Tupala E, Hall H, Bergstrom K, Mantere T, Rasanen P, Sarkioja T, et al. Dopamine D2 receptors and transporters in type 1 and 2 alcoholics measured with human whole hemisphere autoradiography. Hum Brain Mapp. 2003;20:91–102. doi: 10.1002/hbm.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiyatkin EA, Gratton A. Electrochemical monitoring of extracellular dopamine in nucleus accumbens of rats lever-pressing for food. Brain Res. 1994;652:225–234. doi: 10.1016/0006-8993(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 19.Schutz Y, Weinsier R, Hunter GR. Assessment of free-living physical activity in humans: An overview of currently available and proposed new measures. Obes Res. 2001;9:368–379. doi: 10.1038/oby.2001.48. [DOI] [PubMed] [Google Scholar]
- 20.Alsio J, Olszewski P, Norback A, Gunarsson Z, Levine A, Rickering C, et al. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. 2010;171:779–787. doi: 10.1016/j.neuroscience.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure (R)) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18:2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- 23.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross-sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity. 2008;16:119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 26.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: A voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 27.Klein SB, Thorn BM, editors. Biological Psychology. New York: Worth Publishers; 2007. [Google Scholar]
- 28.Pannacciulli N, Le DS, Chen K, Reiman EM, Krakoff J. Relationships between plasma leptin concentrations and human brain structure: a voxel-based morphometric study. Neurosci Lett. 2007;412:248–253. doi: 10.1016/j.neulet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31:1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horstmann A, Busse FP, Mathar D, Muller K, Lepsien J, Schlög H, et al. Obesity-related differences between women and men in brain structure and goal-directed behavior. Front Hum Neurosci. 2011;5:58. doi: 10.3389/fnhum.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitfrontal volume reductions and executive dysfunction. doi: 10.1038/oby.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haltia LT, Viljanen A, Parkkola R, Kemppainen N, Rinne JO, Nuutila P, et al. White matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab. 2007;92:3278–3284. doi: 10.1210/jc.2006-2495. [DOI] [PubMed] [Google Scholar]
- 33.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 35.Doupis J, Rahangdale S, Gnardellis C, Pena S, Mlahotra A, Veves A. Effects of diabetes and obesity on vascular reactivity, inflammatory cytokines, and growth factors. Obesity. 2010;19:729–735. doi: 10.1038/oby.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann M, Ristow M, Boeing H, Pfeiffer A. Inflammatory cytokines and the risk to develop type 2 diabetes. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. (2003). [DOI] [PubMed] [Google Scholar]
- 37.Hu F, Meigs J, Li T, Rifai N, Manson J. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 38.Jefferson A, Massaro J, Wolf P, Seshadri S, Au R, Vasan R, et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68:1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsland A, Gianaros P, Abramowitch S, Manuck S, Hariri A. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;15:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willette A, Bendlin B, McLaren D, Canu E, Kastman E, Kosmatka K, et al. Age-related changes in neural volume and microstructure associated with interleukin-6 are ameliorated by a calorie-restricted diet in old rhesus monkeys. Neuroimage. 2010;51:9870–9994. doi: 10.1016/j.neuroimage.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.You T, Sonntag W, Leng X, Carter C. Lifelong caloric restriction and interleukin-6 secretion from adipose tissue: Effects on physical performance decline in aged rats. J Gerontol A Biol Sci Med Sci. 2007;62:1082–1087. doi: 10.1093/gerona/62.10.1082. [DOI] [PubMed] [Google Scholar]
- 42.Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, et al. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest. 2006;116:1081–1091. doi: 10.1172/JCI26640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattson MP, Duan W, Chan SL, Cheng A, Haughey N, Gary DS, et al. Neuroprotective and neurorestorative signal transduction mechanisms in brain aging: modifications by genes, diet, and behavior. Neurobiol Aging. 2002;23:695–705. doi: 10.1016/s0197-4580(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 44.Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther _targets. 2002;6:601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- 45.Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: The role of impulsivity. Eat Behav. 2006;7:315–322. doi: 10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Pickering AD, Diaz A, Gray JA. Personality and reinforcement: An exploration using a maze learning task. Pers Individ Dif. 1995;18:541–558. [Google Scholar]
- 47.Diergaarde L, Pattij T, Nawijn L, Schoffelmeer AN, Vries TJ. Trait impulsivity predicts escalation of sucrose seeking and hypersensitivity to sucrose-associated stimuli. Behav Neurosci. 2002;123:794–803. doi: 10.1037/a0016504. [DOI] [PubMed] [Google Scholar]
- 48.Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel- based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30:1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batterink L, Yokum S, Stice E. Obese versus lean individuals show reduced inhibitory control in response to food. Neuroimage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCaffery J, Haley A, Sweet L, Phelan S, Raynor H, Parigi A, et al. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr. 2009;90:928–934. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99:538–543. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dietz WH, Robinson TN. Use of body mass index (BMI) as a measure of overweight in children and adolescents. J Pediatr. 1998;132:191–193. doi: 10.1016/s0022-3476(98)70426-3. [DOI] [PubMed] [Google Scholar]
- 53.Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Int J Obes. 2002;75:978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 54.Steinberger J, Jacobs DR, Raatz S, Moran A, Hong C-P, Sinaiko AR. Comparison of body fatness measurements by BMI and skinfolds vs dual energy X-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int J Obes. 2005;29:1346–1352. doi: 10.1038/sj.ijo.0803026. [DOI] [PubMed] [Google Scholar]
- 55.Kuczmarski R, Ogden C, Grummer-Strawn L, Flegal K, Guo S, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;8:1–27. [PubMed] [Google Scholar]
- 56.Smith S. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited-again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 58.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 59.Cohen J. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 60.Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S, et al. Sexual dimorphism of age-realted changes in whole-body fat distribution in obese. In J Obes Relat Metabl Disord. 1994;18:207–202. [PubMed] [Google Scholar]
- 61.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 62.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity. 2006;(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 63.Wandell BA. Computational neuroimaging of human visual cortex. A Rev Neurosci. 1999;10:145–173. doi: 10.1146/annurev.neuro.22.1.145. [DOI] [PubMed] [Google Scholar]
- 64.Kanwisher N, Wojciulik E. VisualAttention:InsightsfromBrainImaging. Nat Rev Neurosci. 2000;1:91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- 65.Van der Laan LN, de Ridder DTD, Viergever MA, Smeets PAM. The first taste is always with the eyes: a meta-analysis on the neural correlates of visual food cues. Neuroimage. 2011;55:296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 66.Ashburner J, Friston K. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- 67.Mechelli A, Price CJ, Friston K, Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Cur Med Imag Rev. 2005;1:1–9. [Google Scholar]
- 68.Del Parigi A, Chen K, Gautier J-F, Salbe AD, Pratley RE, Ravussin E, et al. Sex differences in the human brain’s response to hunger and satiation. Am J Clin Nutr. 2002;75:1017–1022. doi: 10.1093/ajcn/75.6.1017. [DOI] [PubMed] [Google Scholar]
- 69.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 70.Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. Eur J Neurosci. 2004;20:1411–1418. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi M, Tekeda M, Hattori N, Fukanaga M, Sasabe T, Inoue N, et al. Functional imaging of gustatory perception and imagery: ‘top-down’ processing of gustatory signals. Neuroimage. 2004;23:1271–1282. doi: 10.1016/j.neuroimage.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63:652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62:1545–1548. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 75.Sena A, Sarliève LL, Rebel G. Brain myelin of genetically obese mice. J Neurol Sci. 68:233–243. doi: 10.1016/0022-510x(85)90104-2. [DOI] [PubMed] [Google Scholar]
- 76.Menon V, Adleman N, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tatarinni PA, DelParigi A. Functional neuroimaging: a new generation of human brain studies in obesity research. Obes Rev. 2003;4:229–238. doi: 10.1046/j.1467-789x.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 78.McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentic AM. Body fat reference curves for children. Int J Obesity. 2006;30:598–602. doi: 10.1038/sj.ijo.0803232. [DOI] [PubMed] [Google Scholar]
- 79.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]