Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive, fatal disease, thought to be largely transforming growth factor β (TGFβ) driven, for which there is no effective therapy. We assessed the potential benefits in IPF of nitrated fatty acids (NFAs), which are unique endogenous agonists of peroxisome proliferator-activated receptor γ (PPARγ), a nuclear hormone receptor that exhibits wound-healing and antifibrotic properties potentially useful for IPF therapy. We found that pulmonary PPARγ is down-regulated in patients with IPF. In vitro, knockdown or knockout of PPARγ expression in isolated human and mouse lung fibroblasts induced a profibrotic phenotype, whereas treating human fibroblasts with NFAs up-regulated PPARγ and blocked TGFβ signaling and actions. NFAs also converted TGFβ to inactive monomers in cell-free solution, suggesting an additional mechanism through which they may inhibit TGFβ. In vivo, treating mice bearing experimental pulmonary fibrosis with NFAs reduced disease severity. Also, NFAs up-regulated the collagen-_targeting factor milk fat globule-EGF factor 8 (MFG-E8), stimulated collagen uptake and degradation by alveolar macrophages, and promoted myofibroblast dedifferentiation. Moreover, treating mice with established pulmonary fibrosis using NFAs reversed their existing myofibroblast differentiation and collagen deposition. These findings raise the prospect of treating IPF with NFAs to halt and perhaps even reverse the progress of IPF.—Reddy, A. T., Lakshmi, S. P., Zhang, Y., Reddy, R. C. Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages.
Keywords: TGF, collagen, MFG-E8
Idiopathic pulmonary fibrosis (IPF) is a progressive, fatal disease characterized by patchy areas of lung remodeling and fibrosis (1). Progression is associated with active proliferation and transdifferentiation of fibroblasts to myofibroblasts, accompanied by deposition of collagen and other extracellular matrix (ECM) proteins (2). In IPF, myofibroblasts, identifiable histologically by the presence of α-smooth muscle actin (α-SMA), actively secrete ECM and play a crucial role in contributing to the pathophysiology of pulmonary fibrosis. The best available current therapy modestly slows but does not halt progression of the disease, while other therapies that have been used empirically provide no demonstrable benefit (3). Despite best current care, median survival remains poor (<5 yr; ref. 4), and new effective therapeutic strategies are urgently needed.
The main molecular driver of fibrosis is transforming growth factor β (TGFβ; ref. 5), a cytokine that promotes transdifferentiation of myofibroblasts and production of ECM (6). TGFβ is secreted as an inactive latent complex that requires bioconversion for biological activity (5). The monomeric form of TGFβ is inactive and is a competitive inhibitor of the active dimeric form (7). Active TGFβ dimer reacts with its receptor complex to stimulate phosphorylation and activation of SMAD molecules that transmit the signal to the nucleus (8). SMADs then activate or repress gene transcription by binding to SMAD-binding elements (SBEs) in the promoter regions of _target genes. Whether this binding activates or represses transcription appears to depend on cell-specific interactions with other transcription factors that are poorly understood (9). Among the various SMADs having different signaling properties, SMAD2 and SMAD3 form complexes with factors directing them to collagen and other fibrosis-related genes (10) and thus are crucial in this disease.
The nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ) exhibits tissue-protective and wound-healing properties (11), and PPARγ agonists have been shown to exhibit antifibrotic activity in vitro (12) and in a bleomycin-induced model of pulmonary fibrosis (13). These antifibrotic activities reflect blockade of SMAD activation (14). Endogenous nitrated fatty acids (NFAs) are potent PPARγ agonists (15, 16). NFAs are produced by nonenzymatic reaction of unsaturated fatty acids with nitric oxide (NO; ref. 17). The most common NFAs in human plasma are 10-nitro-oleic acid (OA-NO2) and 12-nitrolinoleic acid (LNO2) (15, 18), which exhibit potencies and available concentrations compatible with an important physiological role in regulation of PPARγ activity. NFAs are also electrophiles, capable of undergoing addition to nucleophiles such as thiols via the Michael reaction (19) and thereby modulating the intracellular oxidative state and various signaling pathways. These multiple mechanisms of action suggest that endogenous NFAs may subserve multiple physiological roles and their biological actions may be applicable therapeutically.
Because of the favorable and pulmonary fibrosis-relevant properties of NFAs, here we tested their ability to block the profibrotic effects of TGFβ in vitro and to reduce pulmonary fibrosis in a bleomycin-induced murine model. We found that NFAs acted by novel pathways to not only prevent disease progression but also decrease collagen levels and myofibroblast numbers, thus reversing established fibrosis—an unprecedented treatment outcome with translational implications.
MATERIALS AND METHODS
Patient samples
Human lung tissues were obtained from excess pathological tissue after lung transplantation and organ donation, under a protocol approved by the University of Pittsburgh Institutional Review Board. IPF lung tissues were obtained from explanted lungs of subjects with advanced IPF, and control lungs were donated lungs not suitable for transplantation from the Center for Organ Recovery and Education (CORE). Lung tissues were stored at −80°C until future usage.
Animals
Male C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and were used at 6–8 wk of age (20–25 g). All studies were performed according to protocols reviewed and approved by the Atlanta Veterans Affairs Medical Center Institutional Animal Care and Use Committee.
Fibroblast isolation and culture
Fibroblast cultures were established as described previously (20) from freshly obtained patient lung samples by mincing them in sterile PBS and placing tissue pieces in 100 mm tissue culture dishes containing DMEM supplemented with 10% fetal bovine serum (FBS), 10,000 U/ml penicillin, and 10,000 μg/ml streptomycin (HyClone, Logan, UT, USA) at 37°C in a humidified atmosphere of 5% CO2-95% air. After cells grew out from the explants, they were trypsinized and plated in supplemented DMEM. Cells were used between passages 6 and 10. Human fetal lung fibroblast (IMR-90) cells were obtained from the Coriell Institute for Medical Research (Camden, NJ, USA), and maintained under the same conditions as patient-derived fibroblasts. Monolayer cultures at 90% confluence were deprived of serum for 24 h before treatment with test compounds as indicated.
To generate PPARγ−/− fibroblasts, mice carrying a tamoxifen-inducible Cre-recombinase (Cre-ERT) under the control of a fibroblast-specific regulatory sequence from the procollagen type II, α1 (Col2α1) promoter sequence were crossed with mice that were homozygous for the PPARγ flox allele (Jackson Laboratories), which generated Col2α1-cre+/− PPARγ flox+/− heterozygote mice. The second cross resulted in Col2α1-cre+/+ PPARγ flox+/+ homozygote mice. Mice were then genotyped with specific primers to detect the Cre-ERT and PPARγ flox alleles via a PCR reaction. Mouse lung fibroblasts were isolated as described above, and PPARγ was deleted by treating cells with a stock solution of tamoxifen (4-hydroxitamoxifen; Sigma-Aldrich, St. Louis, MO, USA) as indicated.
Western blotting
Total protein extracts were prepared and Western blotting was performed as described previously (21). Antibodies against PPARγ, α-tubulin, TGFβ receptor 1 (TβR1), p-SMAD2/3, SMAD2/3, and Col1A were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody against p-Ser was from Cell Signaling Technology (Beverly, MA, USA). Antibody against α-SMA was from Dako North America, Inc. (Carpinteria, CA, USA). Antibody against milk fat globule-EGF factor 8 (MFG-E8) was from R&D Systems (Minneapolis, MN, USA). Following primary antibody reaction, the membrane was washed in Tris-buffered saline with Tween 20 (TBST) and incubated with a 1:5000 dilution of secondary antibodies consisting of donkey anti-mouse IR-680 (red) and goat anti-rabbit IR-780 (green), both from LI-COR Biosciences (Lincoln, NE, USA), for 1 h at room temperature. The infrared signal was detected using an Odyssey infrared imager (LI-COR).
RNA isolation and real-time RT-PCR
RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA, USA), and cDNA was generated from 100 ng of total RNA using MultiScribe reverse transcriptase (Applied Biosystems, Foster City, CA, USA) employing random and oligo-dT primers. Real-time PCR was performed using 100 ng cDNA with 2× SYBR Green Master mix (Applied Biosystems) and specific primers for the genes of interest (Supplemental Table S1). These experiments were performed on an AB 7500 fast thermal cycler (Applied Biosystems) using a 3-step protocol employing the melting curve method. The average of each gene cycle threshold (Ct) was determined for each experiment. Relative cDNA levels (2−ΔΔCt) for the genes of interest were determined using the comparative Ct method, which generates the ΔΔCt as the difference between the gene of interest and the housekeeping genes β-actin and 9s rRNA for each sample. Each averaged experimental gene expression sample was compared to the averaged control sample, which was set to 1.
Transcription factor DNA-binding activity assay
Nuclear proteins were extracted using a nuclear extraction kit (Active Motif, Carlsbad, CA, USA), and their concentrations were determined using the BCA Protein Assay kit (Pierce, Rockford, IL, USA). Nuclear extracts were used to quantify DNA-binding activity of PPARγ using an ELISA-based kit (40196; Active Motif).
Transfecting siRNA
Cells were incubated for 8 h with a liposome complex containing siRNA and Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) under serum- and antibiotic-free conditions. The siRNA, either _targeted to the indicated gene (PPARγ, TβR1, or SMAD2/3) or a scrambled control, was obtained from Santa Cruz Biotechnology. After 8 h, fresh medium with 10% FBS was added, and the cells were incubated for a further 16 h. After this 24 h incubation, cells were utilized as described.
Collagen gel contraction assay
Gel contraction assays were performed using a cell contraction assay kit (Cell Biolabs, Inc., San Diego, CA, USA), according to the manufacturer's instructions. Briefly, 0.5 ml of the cell-collagen mixture per well was added into a 24-well plate and incubated for 1 h at 37°C, forming a 3-dimensional collagen gel. The gels were separated from the wells using a sterile pipette tip. The gels were then either untreated or treated with test compounds as indicated. The gels were imaged and weighed after 48 h of treatment. Gel contraction was quantitated as percentage contraction by comparing the weights of different treatment groups with cell-free gels.
Immunoprecipitation
Total protein extracts were prepared and were immunoprecipitated using the Dynabeads Protein G Immunoprecipitation Kit (Invitrogen). Antibodies were bound to Dynabeads Protein G, and the Dynabeads-Ab complex was used to precipitate _target proteins from the total protein extracts. Unbound proteins were washed away and complexes were eluted. All samples (20 μg/lane) were separated by electrophoresis on SDS-polyacrylamide gels and transferred to membranes, and Western blotting was performed.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed using SimpleChIP enzymatic ChIP kit with magnetic beads (Cell Signaling Technology). Briefly, cellular chromatin was crosslinked with 1% formaldehyde for 10 min at room temperature, the crosslinking was stopped with 0.125 M glycine, and cells were washed twice with ice-cold PBS. Nuclei were pelleted and digested by micrococcal nuclease. Following sonication and centrifugation, equal amounts of sheared chromatin were incubated overnight at 4°C with antibodies, IgG as negative control, and RNA polymerase II as positive control (Cell Signaling Technology). Protein G magnetic beads were then added, and the chromatin was incubated with rotation for 2 h at 4°C. An aliquot of chromatin that was not incubated with any antibody was used as the input control sample. Antibody-bound protein/DNA complexes were eluted and subjected to real-time PCR as described above with specific primers for α-SMA, PPARγ, and α-satellite (Supplemental Table S1).
Transient transfection assay
Transient transfection and luciferase activity assays were performed as described previously (22). Briefly, cells were transiently transfected with plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Following treatment with test compounds, activation was measured using the Dual Luciferase Reporter Assay Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions.
Immunofluorescence staining and confocal imaging
Immunofluorescence staining of cultured cells followed by confocal imaging was carried out as described previously (23).
OA-NO2 and bleomycin sulfate administration
Mice were anesthetized with 90 mg/kg ketamine and 10 mg/kg xylazine, administered via intraperitoneal injection, and a tracheotomy was performed. Mice were intratracheally instilled with bleomycin sulfate (Bleo; Sigma-Aldrich) as a single dose of 0.025 U in 50 μl saline. Control mice received 50 μl saline. Following Bleo administration, mice were intratracheally administered, using a small animal intubation system (Kent Scientific Corp., Torrington, CT, USA), 25 μg OA-NO2 (Cayman Chemical, Ann Arbor, MI, USA) in 50 μl of 10% DMSO or received vehicle, daily as indicated. At 24 h following the last drug delivery, the mice were euthanized, bronchoalveolar lavage (BAL) fluid was collected, and the lungs were excised for histopathological examination and measurement of markers of fibrosis.
Lung histopathology
Lungs were inflated and fixed with 10% neutral formalin overnight at room temperature. Lung tissue was dehydrated with increasing ethanol concentrations and then embedded in paraffin. Paraffin sections (5 μm thick) were stained with hematoxylin and eosin (H&E), Masson's trichrome, or picrosirius red. A scoring system was employed as described previously (24). Scores were assigned without knowledge of the treatment involved, and the slides were presented in a random order for each examination.
Measurement of collagen, hydroxyproline, TGFβ, and matrix metalloproteinase 2 (MMP-2) levels in lung
Lung homogenates from different treatment groups were prepared as described previously (21).
Levels of collagen were determined using Sircol collagen assay (Biocolor, Ltd., Belfast, UK). Levels of hydroxyproline were determined using a Biovision hydroxyproline assay kit (Biovision, Milpitas, CA, USA). TGFβ was measured using an ELISA kit (R&D Systems) according to the manufacturer's instructions. MMP-2 activity was measured using the MMP-2 Biotrak activity assay kit (GE Healthcare, Waukesha, WI, USA) according to the manufacturer's instructions.
MFG-E8 expression and collagen uptake
Collagen uptake assays were performed as described previously (25). Briefly, alveolar macrophages (AMs) were isolated and cultured in DMEM supplemented with 10% FBS. Cells were treated with drugs at 37°C in a humidified atmosphere of 5% CO2-95% air as described, and MFG-E8 expression was analyzed by Western blotting. Other AMs were incubated for 60 min in RPMI with 0.1% BSA and 10% mouse serum. Cells were then treated with FITC-conjugated type I collagen (Invitrogen) for 30 min, after which they were washed to remove uningested collagen, counterstained with DAPI, and mounted on glass slides. They were then examined by confocal microscopy.
TGFβ monomer formation
To demonstrate TGFβ monomer formation, human recombinant TGFβ1 (R&D Systems) was incubated with test compounds for the indicated time periods and monomer formation was analyzed by running these samples in a gradient SDS-PAGE gel under nonreducing conditions and performing Western blot analysis using antibody to TGFβ (Santa Cruz Biotechnology).
Molecular modeling and computer simulations of binding of OA-NO2 with TGFβ
Molecular modeling and covalent docking analysis was carried out as described previously (26) using Discovery Studio 2.5 (Accelrys Inc., San Diego, CA, USA).
Statistical analysis
Data are presented as means ± sd. Differences between groups were analyzed using an unpaired t test or ANOVA, followed by a Bonferroni multiple comparison correction. Analyses were performed using GraphPad Prism 5.03 (GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered significant.
RESULTS
PPARγ and TGFβ exhibit negative crosstalk
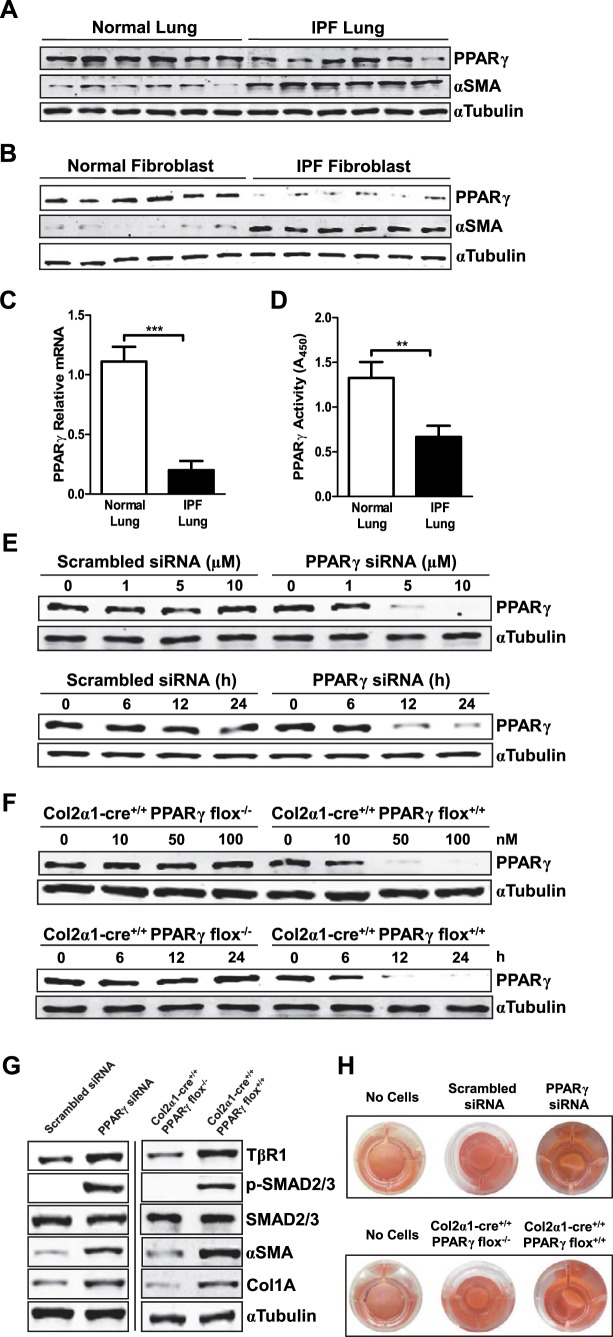
Because recent evidence suggests beneficial actions of PPARγ agonists in IPF, we assessed the potential pathophysiological role of PPARγ in pulmonary fibrosis. We found PPARγ levels both in tissue samples (Fig. 1A) and fibroblasts, the key pathophysiological _target cell in IPF (Fig. 1B), from lungs of patients with IPF were decreased vs. those seen in samples from normal controls. In lung tissues, PPARγ-encoding mRNA transcripts were decreased by >70% (Fig. 1C), and total PPARγ activity (Fig. 1D) was likewise decreased. These data indicate that IPF is associated with decreased PPARγ levels, especially in fibroblasts, accompanied by increased expression of α-SMA, a marker of myofibroblast transdifferentiation (Fig. 1A, B).
Figure 1.
Decreased PPARγ expression and activity, seen in IPF, up-regulates TGFβ signaling pathways and expression of fibrosis-related proteins. A) Tissue extracts from pathologically normal (non-IPF; n=6) and IPF (n=6) lung were subjected to Western blotting for PPARγ and α-SMA, with α-tubulin as a loading control. B) Fibroblasts were isolated from excised lung samples and then were harvested, and whole-cell lysates were subjected to Western blotting for PPARγ and α-SMA. C) PPARγ mRNA in lungs from patients with and without IPF was determined by real-time PCR. D) PPARγ DNA-binding activity in nuclear extracts from lungs of patients with and without IPF. E) IMR-90 human lung fibroblasts were treated with different doses (for 24 h) and for different time periods (with 10 μM) of PPARγ-_targeted siRNA, or scrambled siRNA as a control, after which PPARγ protein in whole-cell extracts was assessed by Western blotting. F) Lung fibroblasts from mice with tamoxifen-inducible, fibroblast-specific PPARγ knockout were treated with tamoxifen with different concentrations (for 24 h) and for different time periods (with 100 nM), after which PPARγ expression in whole-cell lysates was analyzed by Western blotting. G) Human lung fibroblasts were treated with either PPARγ-_targeted siRNA (left panel) or murine fibroblasts with tamoxifen (right panel) to induce PPARγ gene knockout, after which the indicated TGFβ signaling molecules or fibrosis-related proteins were analyzed by Western blotting. H) Lung fibroblasts were treated either with PPARγ-_targeted siRNA (top panel) or with tamoxifen (bottom panel) to induce PPARγ gene knockout, after which gel contraction assays were performed and analyzed. Data are representative of 2–3 independent experiments; n = 3 (for H). **P < 0.01, ***P < 0.001.
To assess whether such down-regulation of PPARγ expression and activity could lead to up-regulation of TGFβ activity and markers of fibrosis, we used a loss-of-function approach. To suppress PPARγ gene expression, we used PPARγ-_targeted siRNA in human lung IMR-90 fibroblast cells (Fig. 1E), and tamoxifen-induced, fibroblast-specific, PPARγ gene knockout in isolated mouse lung fibroblasts (Fig. 1F). With either technique, a >95% reduction of PPARγ compared with baseline levels was seen. We found that even in the absence of exogenous TGFβ these treatments increased type 1 TβR1 levels and phosphorylation of the key TGFβ signaling molecules SMAD2 and SMAD3. PPARγ reduction also increased levels of collagen and the myofibroblast marker α-SMA (Fig. 1G) and, consistent with this, also enhanced myofibroblast transdifferentiation as assayed by a gel contraction assay reflecting the presence of contractile α-SMA (Fig. 1H). The results suggest that the loss of PPARγ function observed in patients with IPF may promote the profibrotic phenotype.
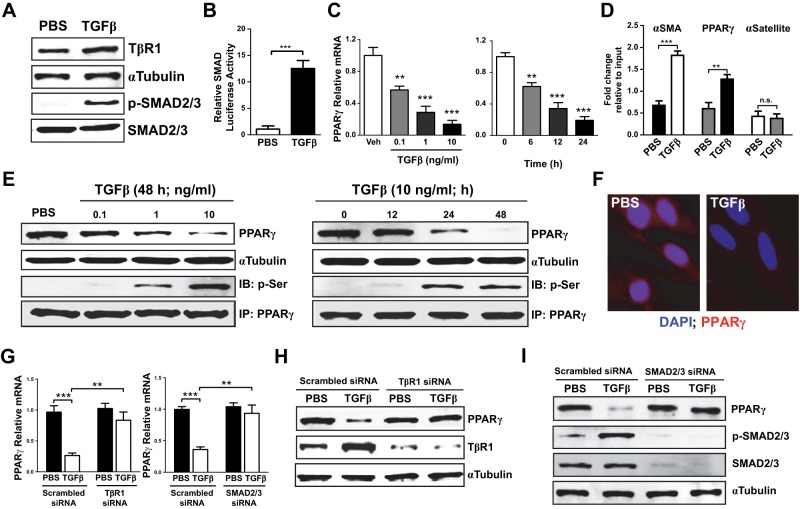
TGFβ down-regulates PPARγ in vitro
Because TGFβ plays a crucial role in the development of pulmonary fibrosis, we hypothesized that it might elicit the PPARγ down-regulation seen in IPF fibroblasts. To test this idea and investigate the molecular mechanisms involved, we assessed responses to TGFβ in cultured human lung fibroblasts. Exposure to TGFβ up-regulated expression of TβR1 and phosphorylation of SMAD2/3 (Fig. 2A) as well as SMAD transcriptional activity (Fig. 2B) accompanied by time- and concentration-dependent decreases in PPARγ transcription (Fig. 2C). ChIP analysis showed that such decreased PPARγ transcription reflected binding of SMAD to a novel SBE in the PPARγ gene's promoter region (Fig. 2D) and also revealed SMAD binding to the α-SMA promoter. TGFβ exposure also decreased PPARγ protein expression in a time- and concentration-dependent manner while increasing the proportion of PPARγ that had undergone inhibitory phosphorylation (Fig. 2E). Decreased PPARγ protein expression was also seen on confocal microscopy (Fig. 2F). Knockdown of TβR1 or of SMAD2/3 abrogated the effects of TGFβ on PPARγ mRNA (Fig. 2G) and protein expression (Fig. 2H, I). These results, together with those shown in Fig. 1, suggest that down-regulation of PPARγ may be a major mechanism by which the profibrotic activity of TGFβ and its key role in IPF pathophysiology are exerted.
Figure 2.
TGFβ down-regulates PPARγ. A) IMR-90 human lung fibroblasts were treated with TGFβ, after which expression of TβR1 and activating phosphorylation of SMAD2/3 were analyzed by Western blotting. B) Lung fibroblasts were transfected with a SMAD reporter construct (Qiagen) before treatment with TGFβ; luciferase activity was measured 24 h later. C) PPARγ mRNA in lung fibroblasts was measured by real-time PCR following TGFβ treatment at different concentrations (for 24 h) and for different time periods. D) Following TGFβ treatment of lung fibroblasts, chromatin was crosslinked and immunoprecipitated (IP) with antibodies to SMAD; the antibody-bound DNA-protein complexes were then subjected to real-time PCR with primers specific for α-SMA and PPARγ promoter regions, thus identifying the presence of SBEs in these promoters; α-satellite was used as control. E) Lung fibroblasts were treated with TGFβ for different time periods and with different doses. Top blot: PPARγ in whole-cell lysates was assessed by Western blotting. Bottom blot: phosphorylation of immunoprecipitated PPARγ was assessed by Western blotting. IB, immunoblot. F) PPARγ expression (red) was assessed by confocal microscopy following treatment of lung fibroblasts with TGFβ (for 48 h). G) PPARγ mRNA was measured by real-time PCR following treatment of lung fibroblasts with TβR1-_targeted (left panel) or SMAD2/3-_targeted (right panel) siRNA before TGFβ treatment. H, I) PPARγ expression in lung fibroblasts was assessed by Western blotting following treatment with TβR1-_targeted (H) or SMAD2/3-_targeted (I) siRNA before TGFβ treatment. Except where indicated, TGFβ was used at a concentration of 2 ng/ml. Data are representative of 3 independent experiments; n = 4–6. **P < 0.01, ***P < 0.001.
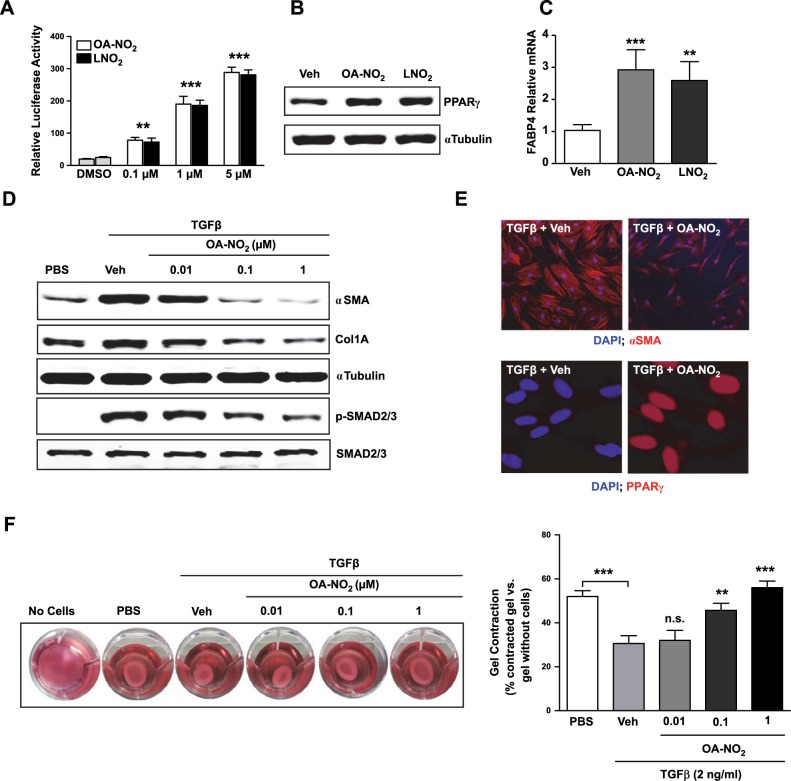
NFAs up-regulate and activate PPARγ and block TGFβ effects in vitro
Because the loss-of-function studies indicated that PPARγ opposes the profibrotic effects of TGFβ, we tested whether NFA-induced up-regulation of PPARγ activity would elicit antifibrotic effects. Treating human lung fibroblasts with either OA-NO2 or LNO2 increased PPARγ activity (Fig. 3A) and protein expression (Fig. 3B), and also elevated transcription of the PPARγ-regulated fatty acid binding protein 4 (FABP4) gene (Fig. 3C). Thus, NFAs are not only activators but also inducers of PPARγ. We used OA-NO2 for all further studies, as it was somewhat more potent than LNO2.
Figure 3.
NFAs up-regulate PPARγ and inhibit TGFβ effects in vitro. A) IMR-90 human lung fibroblasts transfected with a PPAR reporter construct (luciferase gene under control of the peroxisome proliferator response element isolated from the fatty acid transport protein) were treated with designated concentrations of OA-NO2, LNO2, or vehicle; after 24 h, PPARγ activity was measured with a dual luciferase activity kit. B, C) Lung fibroblasts were treated with OA-NO2 or LNO2 (1 μM); whole-cell lysates were subjected to Western blotting for PPARγ (B; 24 h later), or RNA was isolated, and real-time PCR was performed for the PPARγ-regulated FABP4 gene (C; 6 h later). D) Lung fibroblasts were treated with TGFβ (2 mg/ml) followed after 1 h by various concentrations of OA-NO2; after a further 24 h, the indicated Western blots were performed on whole-cell lysates. E) Lung fibroblasts were treated with TGFβ (2 mg/ml) followed after 1 h by OA-NO2 (100 nM); after a further 24 h, α-SMA and PPARγ expression were assessed by confocal microscopy. F) To assess effects on fibroblast contractility, 3-D collagen gels were prepared and released, then treated with TGFβ (2 mg/ml) followed after 1 h by various concentrations of OA-NO2; after a further 24 h, gels were imaged, and percentage contraction was analyzed. Data are representative of 3 independent experiments; n = 4. n.s., nonsignificant. **P < 0.01, ***P < 0.001.
We found that OA-NO2 treatment inhibited TGFβ-induced α-SMA and collagen synthesis in a time- and concentration-dependent manner, accompanied by reduced SMAD2/3 activation (Fig. 3D). We also confirmed by confocal imaging that OA-NO2 treatment increased expression of PPARγ and decreased that of α-SMA (Fig. 3E). OA-NO2 treatment also concentration dependently inhibited TGFβ-induced myofibroblast transdifferentiation measured by gel contraction assays (Fig. 3F), in which 1 μM OA-NO2 completely blocked TGFβ effects. Together, these results with OA-NO2 further support the conclusion that PPARγ and its activating ligands may significantly influence IPF pathophysiology.
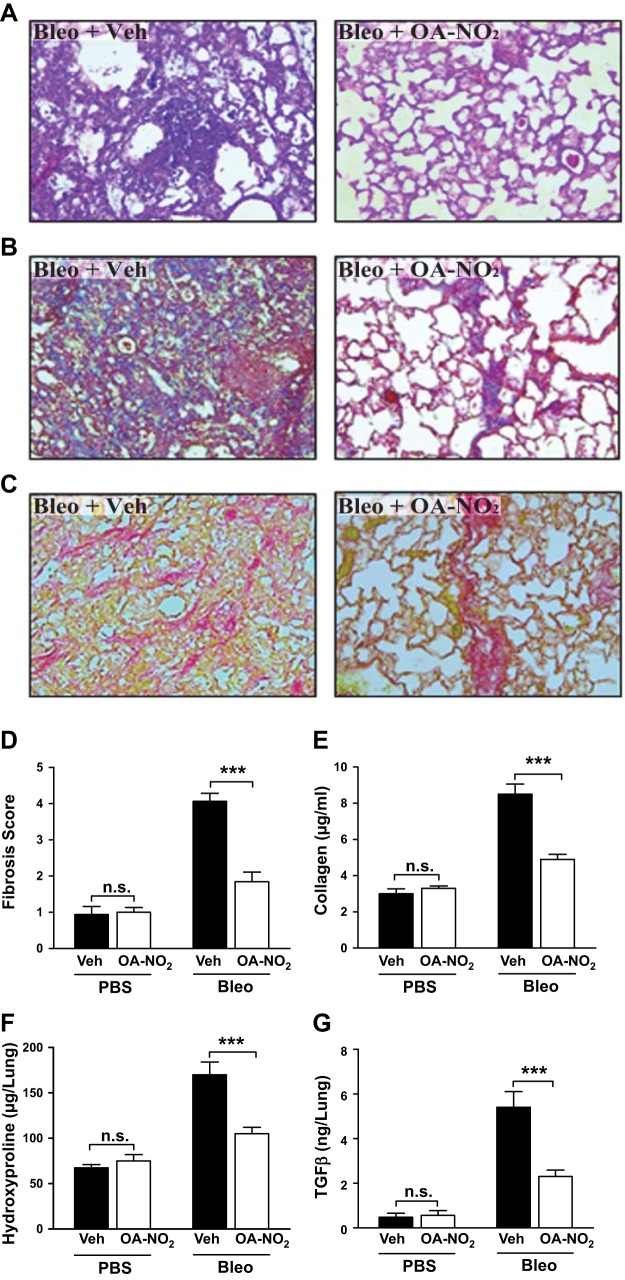
NFAs block bleomycin-induced pulmonary fibrosis in vivo
Our finding that NFAs inhibit the profibrotic effects of TGFβ in lung fibroblasts in vitro suggested that they might prevent pulmonary fibrosis in vivo. We tested this hypothesis by treating murine experimental pulmonary fibrosis with OA-NO2. We induced pulmonary fibrosis in mice by intratracheal administration of bleomycin. Because bleomycin-induced inflammation largely recedes by 11 d posttreatment (27), we initiated intratracheal treatment with OA-NO2 on d 11–20 postbleomycin in order to assess potential antifibrotic rather than earlier anti-inflammatory effects. Lungs were excised for analysis 24 h after the final OA-NO2 delivery.
The results showed that OA-NO2 treatment elicited major reductions in bleomycin-induced histopathological changes in lung architecture and collagen content, seen by staining with H&E, Masson's trichrome, and picrosirius red (Fig. 4A–C), and significant reductions in quantitative fibrosis score (Fig. 4D). OA-NO2-induced reductions in fibrosis were also corroborated biochemically, by decreased collagen (Fig. 4E) and hydroxyproline (Fig. 4F) content of lung homogenates, not seen in treated nonfibrotic control mice. OA-NO2 also decreased MMP-2 activity (Supplemental Fig. S2) and TGFβ concentrations in lung homogenates of bleomycin-treated mice (Fig. 4G), suggesting that NFAs may act in part by regulating these important profibrotic molecules.
Figure 4.
NFAs block bleomycin-induced pulmonary fibrosis. Pulmonary fibrosis was induced in mice by a single intratracheal injection of bleomycin (0.025 U) in saline (50 μl). After 10 d, mice received OA-NO2 intratracheally (25 μg in 50 μl of 10% DMSO) or received vehicle daily for 10 d. After a further 24 h, mice were euthanized, BAL fluid was collected, and lungs were excised. A–C) Lung sections were stained with H&E (A), Masson's trichrome (B), or picrosirius red (C) and examined microscopically. D) Fibrosis score was calculated as described. E–G) Lungs were homogenized, and content of collagen (E), hydroxyproline (F), TGFβ (G), and MMP-2 activity (Supplemental Fig. S2) was determined. Original view ×20. Data are representative of 2 independent experiments with n = 9 mice/group (3 mice/group utilized for histology). n.s., nonsignificant. ***P < 0.001.
NFA treatment reverses bleomycin-induced fibrosis
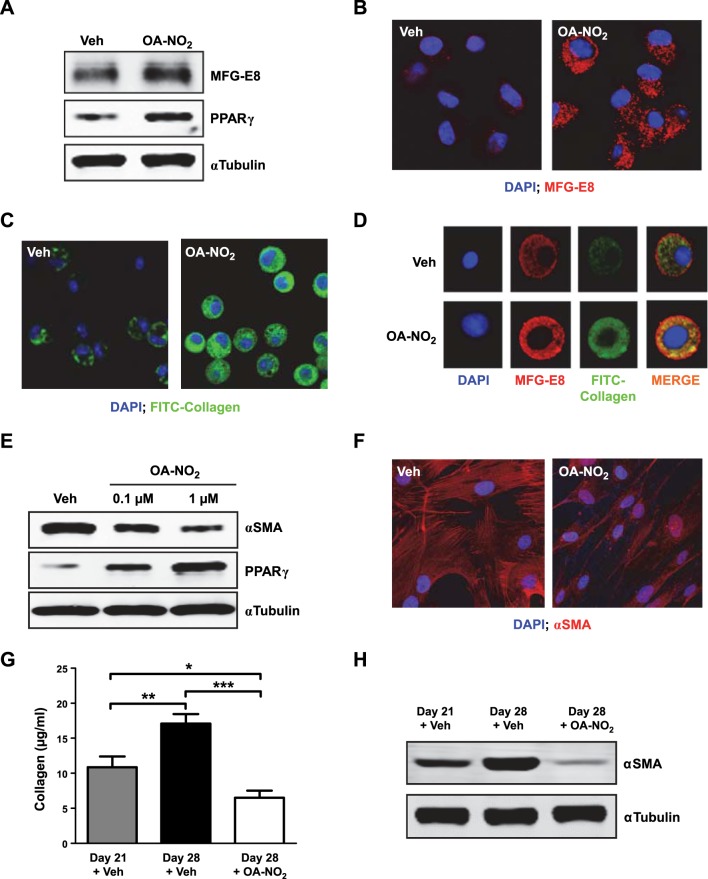
The pronounced reduction in bleomycin-induced fibrosis we saw following even substantially delayed OA-NO2 treatment suggested that the improvement elicited by NFA treatment might include reversal of fibrosis, in addition to cessation of fibrotic progression. Accordingly, we tested this therapeutically important hypothesis in vitro and in vivo. In vitro, we assessed the ability of NFAs to up-regulate MFG-E8, a glycoprotein factor that _targets collagen for uptake and lysosomal degradation by macrophages (25). In isolated alveolar macrophages, OA-NO2 treatment up-regulated expression of PPARγ (Fig. 5A) and MFG-E8 (Fig. 5A, B) as well as collagen uptake (Fig. 5C). Collagen uptake and MFG-E8 expression were colocalized within the same cells (Fig. 5D). We also tested the ability of NFAs to induce dedifferentiation of myofibroblasts, using isolated cultured lung fibroblasts of patients with IPF, which expressed high baseline α-SMA levels. In these cells, OA-NO2 treatment up-regulated PPARγ expression while decreasing myofibroblast differentiation as assessed by α-SMA levels detected by Western blotting (Fig. 5E) and confocal imaging (Fig. 5F).
Figure 5.
NFAs reverse fibrotic changes in vitro and in vivo. A–D) Murine alveolar macrophages were treated with OA-NO2 (1 μM) for 24 h. A) MFG-E8 and PPARγ in whole-cell lysates were assessed by Western blotting. B) MFG-E8 expression (red) was evaluated by confocal microscopy. C) Cells were incubated for 30 min with FITC-conjugated type 1 collagen, and uptake was evaluated by confocal microscopy. D) Images demonstrating collagen uptake and MFG-E8 expression were merged. E, F) Fibroblasts derived from lungs of patients with IPF (n=6) were incubated with OA-NO2 (1 μM for confocal) for 24 h, after which α-SMA and PPARγ in whole-cell lysates were assessed by Western blotting (E), and α-SMA expression (red) was assessed by confocal microscopy (F); representative images from a single patient with IPF are shown. G, H) Pulmonary fibrosis was induced in mice by a single intratracheal injection of bleomycin (0.025 U) in saline (50 μl). Beginning 21 d later and continuing daily for 7 d, mice received OA-NO2 (25 μg) intratracheally. Before initiation of OA-NO2 treatment and 24 h after the final OA-NO2 administration lung collagen content (G) was measured, and α-SMA (H) was assessed by Western blotting. Data are representative of 2–3 independent experiments; n = 6 mice/group (G, H); AMs from 1–3 mice for each treatment group (A–D). *P < 0.05, **P < 0.01, ***P < 0.001.
These findings indicated that NFAs elicit multiple activities potentially capable of reversing established pulmonary fibrosis. To test this idea we assessed whether NFA treatment can reverse fibrosis in vivo by assessing fibrosis markers after administering OA-NO2 to mice with bleomycin-induced pulmonary fibrosis. Mice were treated for 7 d beginning 21 d postbleomycin. The levels of collagen (Fig. 5G) and α-SMA (Fig. 5H) measured in lungs of OA-NO2-treated mice were significantly reduced compared with either baseline levels (at treatment initiation) or those in vehicle-treated controls. These results demonstrate that NFAs can reverse collagen deposition and associated myofibroblast transdifferentiation in a pulmonary fibrosis model, both in vitro and in vivo.
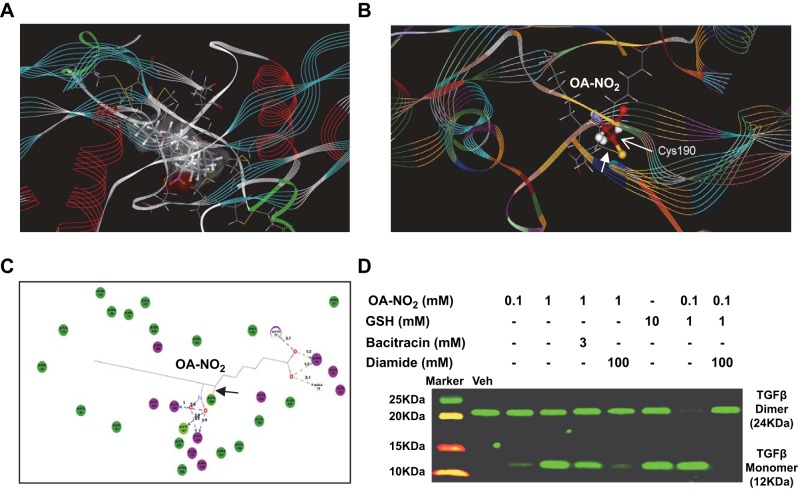
NFAs convert TGFβ dimers to inactive monomers in a cell-free system
The active form of TGFβ is a dimer that can be converted to an inactive monomer by thiol disruption of its disulfide bond (7, 28). We hypothesized that as electrophiles, NFAs might be capable of disrupting the disulfide bond of TGFβ by temporary addition of the monomeric thiol to the NFA electrophilic center. If so, such conversion of TGFβ to inactive monomers would represent an additional, novel mechanism of antifibrotic NFA action. We first tested this hypothesis by in silico modeling (Fig. 6A–C), which supported the idea by revealing multiple interactions between OA-NO2 and TGFβ1 monomer, including covalent bonding to the Cys sulfur atom involved in the disulfide bond. To experimentally confirm this predicted OA-NO2-induced conversion of TGFβ1 to inactive monomers, we incubated TGFβ1 with OA-NO2 and/or the thiol glutathione (GSH) for 30 min, then analyzed the mixture by Western blotting under nonreducing conditions. Exposure of TGFβ1 to OA-NO2 (1 mM) or GSH (10 mM) yielded a dramatic induction of monomeric TGFβ1, and the combined exposure to 0.1 mM OA-NO2 and 1 mM GSH almost completely depleted the dimer form (Fig. 6D). At a concentration of 0.1 mM, OA-NO2 alone yielded a small but discernable amount of monomeric TGFβ1. Bacitracin, a dithiol that inhibits disulfide exchange, reduced monomer production by ∼50%, perhaps by competing for OA-NO2. Effects of 1 mM OA-NO2 or the 0.1 mM OA-NO2-1 mM GSH combination were largely or completely blocked by the oxidizing agent diamide. Such conversion of TGFβ to inactive monomers thus constitutes an additional mechanism by which NFAs may act to block its profibrotic effects.
Figure 6.
NFAs convert TGFβ to inactive monomer. A–C) Binding of OA-NO2 to TGFβ monomer was modeled in silico. A) Solvent accessibility representation. B) Schematic representation showing covalent interaction of TGFβ Cys residue with OA-NO2. C) Ligand binding map generated using Discovery Studio. Covalent bond is indicated by arrowhead (B, C); hydrogen bonds are indicated by dotted lines (C). D) TGFβ (2 mg/ml) was incubated for 30 min with the indicated compounds; diamide, when used, was added at the end of 30 min incubation, with incubation then continued for an additional 30 min. Following incubation, TGFβ monomer and dimer in test samples were assessed by Western blotting under nonreducing conditions. Data are representative of 3 independent experiments.
DISCUSSION
Our findings reveal novel antifibrotic actions of NFAs, both in human fibroblasts in vitro and in a murine model of pulmonary fibrosis. Together, the results suggest that these effects are likely exerted via activation of PPARγ, which we found to be down-regulated in lungs and fibroblasts of patients with IPF. We found that PPARγ deficiency in otherwise normal fibroblasts produced a profibrotic phenotype even in the absence of exogenous TGFβ induction, and we found that NFAs act as both agonists and inducers of PPARγ in fibroblasts. We also discovered a mutually antagonistic crosstalk between PPARγ and TGFβ signaling pathways that likely contributes to the profibrotic influence of PPARγ deficiency in IPF and the antifibrotic effects of PPARγ activation. The antifibrotic actions of NFAs appear highly relevant to IPF, because treating pulmonary fibrosis-bearing mice with NFAs reduced and even reversed established disease, a promising discovery with high translational potential.
The results of our loss-of-function studies substantially extend prior findings that activating PPARγ alleviates pulmonary fibrosis and blocks TGFβ effects in vitro (29), as we found that genetic knockout or knockdown in otherwise normal murine or human lung fibroblasts directly elicits a profibrotic phenotype encompassing both enhanced TGFβ signaling and transdifferentiation into myofibroblasts. We also found that TGFβ down-regulates PPARγ expression in fibroblasts in vitro, raising the possibility that such PPARγ down-regulation may be a key profibrotic mechanism contributing to IPF pathogenesis.
The TGFβ-induced PPARγ down-regulation we saw is analogous to that seen in vascular smooth muscle cells (30), in which the effect was mediated via SMAD and AP-1 activation. In this connection, our ChIP studies revealed a novel SBE within the PPARγ promoter, as also recently found in hepatic stellate cells (31). The presence of such SBEs implies that activated SMAD may repress PPARγ transcription directly. As we saw presently in lung fibroblasts, disrupting TGFβ signaling also increased PPARγ expression (30). Together, this evidence raises the possibility that negative crosstalk between PPARγ and TGFβ may be a common regulatory mechanism in vivo.
NFAs may also act to suppress TGFβ action by reducing its bioavailability, as we found reduced TGFβ levels in lung tissue of NFA-treated mice and also found that NFAs cause conversion of TGFβ from its active dimer to its inactive and even inhibitory (7) monomeric form in a cell-free system. Such NFA-induced reduction in bioavailable TGFβ levels may thus provide an independent, complementary mechanism of NFA-induced suppression of TGFβ signaling.
We found that NFA treatment not only blocked development of pulmonary fibrosis but actually reversed it once established. These effects occurred despite withholding treatment initiation until after initial inflammation had subsided, indicating that NFAs did not act by inhibiting early inflammatory responses and further suggesting that NFAs elicited resorption, degradation, and removal of established fibrotic components. We found that NFA treatment of experimental pulmonary fibrosis produced absolute reductions from pretreatment levels of both pulmonary collagen and the myofibroblast marker α-SMA. Although prior studies have shown reversal of renal (32, 33) and muscle (34) fibrosis (via bone morphogenic protein-7 treatment and suppression of myostatin activation, respectively), and the liver has some intrinsic fibrosis-clearing ability, our finding in lung fibrosis is unprecedented and suggests that NFAs might be useful therapeutically in IPF.
Investigating the mechanisms of NFA-induced collagen removal, we found that NFAs up-regulated expression within AMs of MFG-E8, a factor previously shown to _target collagen for uptake and lysosomal degradation (25). Likewise, NFA-induced up-regulation of MFG-E8 in AMs increased their uptake of collagen. Because NFAs are structurally and functionally distinct from the hormones (prolactin, insulin, and steroids) previously known to stimulate MFG-E8 production (35), it will be important to elucidate the mechanism of this NFA action. Moreover, treating myofibroblasts from patients with IPF with NFAs reduced their α-SMA expression and contractility, implying reversal of their transdifferentiated phenotype. Such transdifferentiation of myofibroblasts had traditionally been considered irreversible, but recent studies found that reversal can be elicited by PGE2 (36) and the Nrf2 inducer sulforaphane (37). This key cell phenotype-switching aspect of NFA action lends further strong support to their potential promise as a means of mechanism-based IPF therapy with sustained effects.
Most pulmonary diseases, such as asthma and chronic obstructive pulmonary disease (COPD), are preferentially treated by inhaled drugs that can be delivered directly to their desired site of action, thus maximizing local dosage and minimizing undesired systemic affects. This is not currently true for IPF, however. NFAs can be readily derivatized by reaction with thiols such as GSH to form water-soluble adducts, and aqueous formulations of these adducts can be nebulized to produce a therapeutically appropriate range of droplet sizes (Supplemental Fig. S1). Inhalation has been shown to be a viable method for drug delivery in IPF (38), further supporting the advantages of NFAs as inhaled treatments.
In summary, we found that NFAs, a unique class of endogenous agents, exert antifibrotic activity and block the profibrotic effects of TGFβ by activating and up-regulating PPARγ and also by reducing available levels of active TGFβ. Treatment with NFAs actively reversed established pulmonary fibrosis in mice by stimulating uptake of lung collagen and reversing myofibroblast transdifferentiation. The potencies of NFAs as PPARγ agonists are similar to those of current synthetic agents, but as endogenous compounds they may prove safer than the synthetic PPARγ agonists currently used for treating type 2 diabetes. Together, these findings carry promise for developing a new therapeutic intervention for human pulmonary fibrosis that might provide curative, rather than merely progression-halting, treatment of this fatal disease.
Supplementary Material
Acknowledgments
This work was supported by a Merit Review Award from the U.S. Department of Veterans Affairs and by U.S. National Institutes of Health grant HL-093196 (to R.C.R).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- α-SMA
- α-smooth muscle actin
- AM
- alveolar macrophage
- BAL
- bronchoalveolar lavage
- ChIP
- chromatin immunoprecipitation
- ECM
- extracellular matrix
- FABP4
- fatty acid binding protein 4
- FBS
- fetal bovine serum
- GSH
- glutathione
- H&E
- hematoxylin and eosin
- IPF
- idiopathic pulmonary fibrosis
- LNO2
- 12-nitrolinoleic acid
- MFG-E8
- milk fat globule-EGF factor 8
- MMP-2
- matrix metalloproteinase 2
- NFA
- nitrated fatty acid
- OA-NO2
- 10-nitro-oleic acid
- PPARγ
- peroxisome proliferator-activated receptor γ
- SBE
- SMAD-binding element
- TβR1
- transforming growth factor β receptor 1
- TGFβ
- transforming growth factor β
REFERENCES
- 1. King T. E., Jr., Pardo A., Selman M. (2011) Idiopathic pulmonary fibrosis. Lancet 378, 1949–1961 [DOI] [PubMed] [Google Scholar]
- 2. Gross T. J., Hunninghake G. W. (2001) Idiopathic pulmonary fibrosis. N. Engl. J. Med. 345, 517–525 [DOI] [PubMed] [Google Scholar]
- 3. Richeldi L. (2012) Assessing the treatment effect from multiple trials in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 21, 147–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Thoracic Society; European Respiratory Society (2002) American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 165, 277–304 [DOI] [PubMed] [Google Scholar]
- 5. Leask A., Abraham D. J. (2004) TGF-beta signaling and the fibrotic response. FASEB J. 18, 816–827 [DOI] [PubMed] [Google Scholar]
- 6. Willis B. C., Borok Z. (2007) TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L525–L534 [DOI] [PubMed] [Google Scholar]
- 7. Lichtenberger F. J., Montague C., Hunter M., Frambach G., Marsh C. B. (2006) NAC and DTT promote TGF-beta1 monomer formation: demonstration of competitive binding. J. Inflamm. (Lond.) 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi Y., Massague J. (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- 9. Massague J., Seoane J., Wotton D. (2005) Smad transcription factors. Genes Dev. 19, 2783–2810 [DOI] [PubMed] [Google Scholar]
- 10. Verrecchia F., Mauviel A. (2007) Transforming growth factor-β and fibrosis. World J. Gastroenterol. 13, 3056–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michalik L., Wahli W. (2006) Involvement of PPAR nuclear receptors in tissue injury and wound repair. J. Clin. Invest. 116, 598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burgess H. A., Daugherty L. E., Thatcher T. H., Lakatos H. F., Ray D. M., Redonnet M., Phipps R. P., Sime P. J. (2005) PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L1146–L1153 [DOI] [PubMed] [Google Scholar]
- 13. Milam J. E., Keshamouni V. G., Phan S. H., Hu B., Gangireddy S. R., Hogaboam C. M., Standiford T. J., Thannickal V. J., Reddy R. C. (2008) PPAR-γ agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L891–L901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao C., Chen W., Yang L., Chen L., Stimpson S. A., Diehl A. M. (2006) PPARγ agonists prevent TGFβ1/Smad3-signaling in human hepatic stellate cells. Biochem. Biophys. Res. Commun. 350, 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baker P. R. S., Lin Y., Schopfer F. J., Woodcock S. R., Groeger A. L., Batthyany C., Sweeney S., Long M. H., Iles K. E., Baker L. M. S., Branchaud B. P., Chen Y. E., Freeman B. A. (2005) Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J. Biol. Chem. 280, 42464–42475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schopfer F. J., Lin Y., Baker P. R. S., Cui T., Garcia-Barrio M., Zhang J., Chen K., Chen Y. E., Freeman B. A. (2005) Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor γ ligand. Proc. Natl. Acad. Sci. U. S. A. 102, 2340–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Donnell V. B., Eiserich J. P., Bloodsworth A., Chumley P. H., Kirk M., Barnes S., Darley-Usmar V. M., Freeman B. A. (1999) Nitration of unsaturated fatty acids by nitric oxide-derived reactive species. Methods Enzymol. 301, 454–470 [DOI] [PubMed] [Google Scholar]
- 18. Baker P. R. S., Schopfer F. J., Sweeney S., Freeman B. A. (2004) Red cell membrane and plasma linoleic acid nitration products: synthesis, clinical identification, and quantitation. Proc. Natl. Acad. Sci. U. S. A. 101, 11577–11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baker L. M., Baker P. R., Golin-Bisello F., Schopfer F. J., Fink M., Woodcock S. R., Branchaud B. P., Radi R., Freeman B. A. (2007) Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J. Biol. Chem. 282, 31085–31093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X. M., Zhang Y., Kim H. P., Zhou Z., Feghali-Bostwick C. A., Liu F., Ifedigbo E., Xu X., Oury T. D., Kaminski N., Choi A. M. (2006) Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J. Exp. Med. 203, 2895–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reddy A. T., Lakshmi S. P., Kleinhenz J. M., Sutliff R. L., Hart C. M., Reddy R. C. (2012) Endothelial cell peroxisome proliferator-activated receptor gamma reduces endotoxemic pulmonary inflammation and injury. J. Immunol. 189, 5411–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Narala V. R., Smith M. R., Adapala R. K., Ranga R., Panati K., Moore B. B., Leff T., Reddy V. D., Kondapi A. K., Reddy R. C. (2009) Curcumin is not a ligand for peroxisome proliferator-activated receptor-gamma. Gene Ther. Mol. Biol. 13, 20–25 [PMC free article] [PubMed] [Google Scholar]
- 23. Lakshmi S. P., Reddy A. T., Naik M. U., Naik U. P., Reddy R. C. (2012) Effects of JAM-A deficiency or blocking antibodies on neutrophil migration and lung injury in a murine model of ALI. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L758–L766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu L., Dong X. W., Shen L. L., Li F. F., Jiang J. X., Cao R., Yao H. Y., Shen H. J., Sun Y., Xie Q. M. (2012) Simvastatin delivery via inhalation attenuates airway inflammation in a murine model of asthma. Int. Immunopharmacol. 12, 556–564 [DOI] [PubMed] [Google Scholar]
- 25. Atabai K., Jame S., Azhar N., Kuo A., Lam M., McKleroy W., Dehart G., Rahman S., Xia D. D., Melton A. C., Wolters P., Emson C. L., Turner S. M., Werb Z., Sheppard D. (2009) Mfge8 diminishes the severity of tissue fibrosis in mice by binding and _targeting collagen for uptake by macrophages. J. Clin. Invest. 119, 3713–3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reddy A. T., Lakshmi S. P., Reddy R. C. (2012) The nitrated fatty acid 10-nitro-oleate diminishes severity of lps-induced acute lung injury in mice. PPAR Res. 2012, 617063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moeller A., Ask K., Warburton D., Gauldie J., Kolb M. (2008) The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 40, 362–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blakytny R., Erkell L. J., Brunner G. (2006) Inactivation of active and latent transforming growth factor beta by free thiols: potential redox regulation of biological action. Int. J. Biochem. Cell Biol. 38, 1363–1373 [DOI] [PubMed] [Google Scholar]
- 29. Sime P. J. (2008) The antifibrogenic potential of PPARgamma ligands in pulmonary fibrosis. J. Invest. Med. 56, 534–538 [DOI] [PubMed] [Google Scholar]
- 30. Fu M., Zhang J., Lin Y., Zhu X., Zhao L., Ahmad M., Ehrengruber M. U., Chen Y. E. (2003) Early stimulation and late inhibition of peroxisome proliferator-activated receptor gamma (PPAR gamma) gene expression by transforming growth factor beta in human aortic smooth muscle cells: role of early growth-response factor-1 (Egr-1), activator protein 1 (AP1) and Smads. Biochem. J. 370, 1019–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng S., Chen A. (2007) Disruption of transforming growth factor-β signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-γ in rat hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G113–G123 [DOI] [PubMed] [Google Scholar]
- 32. Sugimoto H., LeBleu V. S., Bosukonda D., Keck P., Taduri G., Bechtel W., Okada H., Carlson W., Jr., Bey P., Rusckowski M., Tampe B., Tampe D., Kanasaki K., Zeisberg M., Kalluri R. (2012) Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat. Med. 18, 396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tampe B., Tampe D., Muller C. A., Sugimoto H., Lebleu V., Xu X., Muller G. A., Zeisberg E. M., Kalluri R., Zeisberg M. (2014) Tet3-mediated hydroxymethylation of epigenetically silenced genes contributes to bone morphogenic protein 7-induced reversal of kidney fibrosis. J. Am. Soc. Nephrol. 25, 905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bo Li Z., Zhang J., Wagner K. R. (2012) Inhibition of myostatin reverses muscle fibrosis through apoptosis. J. Cell Sci. 125, 3957–3965 [DOI] [PubMed] [Google Scholar]
- 35. Kusunoki R., Ishihara S., Aziz M., Oka A., Tada Y., Kinoshita Y. (2012) Roles of milk fat globule-epidermal growth factor 8 in intestinal inflammation. Digestion 85, 103–107 [DOI] [PubMed] [Google Scholar]
- 36. Garrison G., Huang S. K., Okunishi K., Scott J. P., Kumar Penke L. R., Scruggs A. M., Peters-Golden M. (2013) Reversal of myofibroblast differentiation by prostaglandin E(2). Am. J. Respir. Cell Mol. Biol. 48, 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Artaud-Macari E., Goven D., Brayer S., Hamimi A., Besnard V., Marchal-Somme J., Ali Z. E., Crestani B., Kerdine-Romer S., Boutten A., Bonay M. (2013) Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid. Redox Signal. 18, 66–79 [DOI] [PubMed] [Google Scholar]
- 38. Diaz K. T., Skaria S., Harris K., Solomita M., Lau S., Bauer K., Smaldone G. C., Condos R. (2012) Delivery and safety of inhaled interferon-gamma in idiopathic pulmonary fibrosis. J. Aerosol. Med. Pulm. Drug Deliv. 25, 79–87 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.