Abstract
Type 2 diabetes mellitus is characterized by the dysregulation of glucose homeostasis resulting in hyperglycemia. Although current diabetes treatments have exhibited some success in lowering blood glucose, their effect is not always sustained and their use may be associated with undesirable side effects, such as hypoglycemia. Novel diabetic drugs, which may be used in combination with existing therapies, are therefore needed. The potential of specifically _targeting the liver in order to normalize blood glucose levels has not been fully exploited. Here, we review the molecular mechanisms controlling hepatic gluconeogenesis and glycogen storage, and assess the prospect of therapeutically _targeting associated pathways to treat type 2 diabetes.
Introduction
Type 2 diabetes is the seventh leading cause of death in the United States and affects more than an estimated 29.1 million Americans, with 1.7 million new diagnoses per year1. Additionally, more than 86 million Americans are considered pre-diabetic. Worldwide, this disease is found in 9% of the adult population and directly causes at least 1.5 million deaths annually. Moreover, diabetes significantly increases comorbidities of several other chronic health problems, including cardiovascular disease, stroke, and kidney disease, which contribute heavily to the diabetes health and cost burden2. The debilitating and chronic nature of type 2 diabetes requires effective and long-lasting drug treatments.
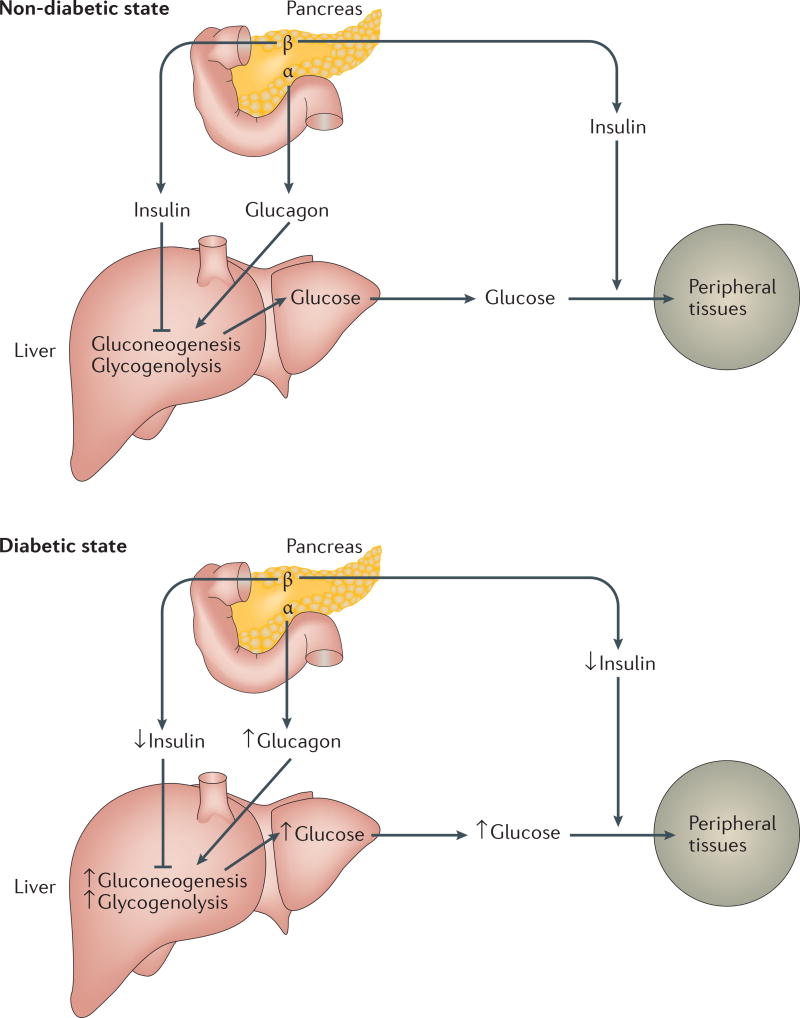
Therapies for type 2 diabetes must ameliorate its pathophysiology, the hallmark of which is decreased insulin secretion and/or insulin insensitivity3. In normal individuals, insulin is secreted by the pancreas to decrease glucose production and increase uptake of blood glucose into peripheral tissues such as skeletal muscle and adipose tissue (Figure 1). In diabetes, decreased insulin release and/or suppressed insulin action leads to increased glucose production and decreased glucose uptake by peripheral tissues, resulting in elevated blood glucose levels.
Figure 1. Schematic of glucose homeostasis in non-diabetic and diabetic states.
After feeding, pancreatic beta cells release insulin to inhibit gluconeogenesis and glycogenolysis in the liver, decreasing glucose output to the circulation. Insulin also acts at peripheral tissues to increase glucose uptake, resulting in decreased blood glucose. During fasting, pancreatic alpha cells release glucagon to increase gluconeogenesis and glycogenolysis in the liver, increasing circulating blood glucose. In the diabetic state, insulin action is decreased at the liver and/or peripheral tissues and glucagon action is enhanced, leading to increased hepatic gluconeogenesis and glycogenolysis, increased glucose release to the circulation, repressed glucose uptake into peripheral tissues, and increased blood glucose levels.
Although several existing type 2 diabetes drugs lower blood glucose levels - including metformin4, sulfonylureas5, glucagon-like peptide 1 (GLP-1) agonists6, glitazones/thiazolidinediones (TZDs)7, alpha-glucosidase inhibitors8, sodium-glucose co-transporter 2 (SGLT2) inhibitors9, and bile acid sequestrants10 (Box 1) - these therapies each have their limitations and drawbacks. In particular, the most widely used drug, metformin, although known to decrease hepatic gluconeogenesis, does not have a well-defined molecular _target4, and is associated with gastrointestinal side effects11. Other classes of drugs are also accompanied by side effects, and may cause hyperinsulinemia, sometimes resulting in hypoglycemia12–14. Novel therapeutic approaches are therefore warranted.
Box 1: Current type 2 diabetes drugs.
The most commonly used diabetes therapy is metformin (N,N-dimethylbiguanide), a biguanide compound that decreases gluconeogenesis237. Its popularity stems from its ability to lower blood glucose without inducing hypoglycemia or weight gain, while maintaining an excellent safety profile4. However, the molecular mechanism of metformin has not been well defined. A generally acknowledged site of action of metformin is the mitochondria, where it partially inhibits complex I238,239 to decrease cellular energy and gluconeogenesis240. How a decrease in cellular energy (as represented by an increase in the AMP:ATP ratio) causes a change in gluconeogenesis is unclear. Some reports have indicated that activation of AMP-activated protein kinase (AMPK) is necessary241. Others have found that AMPK is not needed, but rather that accumulation of AMP:ATP directly inhibits gluconeogenic flux240 and inhibits adenylyl cyclase to decrease cAMP and activation of protein kinase A (PKA)242. Metformin has also been reported to inhibit mitochondrial glycerophosphate dehydrogenase (mGPD), which blocks the glycerophosphate shuttle and alters the hepatic redox state to decrease the conversion of lactate and pyruvate to glucose and therefore decrease gluconeogenesis243. In addition to affecting gluconeogenesis, metformin also decreases tissue lipid storage through AMPK phosphorylation and inactivation of acetyl-coA carboxylase (ACC), which then can improve insulin sensitivity and decrease blood glucose levels244. Although considered safe, metformin is accompanied by gastrointestinal side effects including nausea, which may result from its effects on multiple tissues11. Additionally, the effect of metformin on glycemic control is typically reduced over time, requiring combined treatment with other drugs75.
Several other classes of drugs affect insulin secretion from the pancreas or uptake of glucose into tissues. Sulfonylureas and meglitinides/D-phenylalanine increase insulin secretion by closing KATP channels in pancreatic beta cells5,245. While effective at lowering blood glucose, these agents can cause hypoglycemia, epithelial damage, or beta cell exhaustion or apoptosis12. Glucagon-like peptide 1 (GLP-1) is a gut-secreted hormone that stimulates insulin and impairs glucagon secretion, and its action can be increased through direct agonism or by inhibition of dipeptidyl peptidase-4 (DPP-4), which leads to enhanced GLP-1 secretion6. Side effects of GLP-1 agonists include nausea, diarrhea, and headaches, while DPP-4 inhibitors may cause upper respiratory tract infections and headaches246. Aside from the potential side effects, the insulin-stimulating action of sulfonylureas and GLP-1 agonists may not be the best course of treatment for diabetes, as this can result in weight gain, and hyperinsulinemia has been associated with comorbidities including cardiovascular disease and cancer13,14. However, no substantial link has yet been found between the use of these drugs and the incidence of these comorbidities.
The glitazone and thiazolidinedione (TZD) class of drugs act as agonists for PPARϒ in order to increase glucose uptake into peripheral tissues, thereby increasing insulin sensitivity7. While often effective, this drug class has potential complications including increased risks of myocardial infarction, skeletal fractures, and bladder cancer7. Alpha-glucosidase inhibitors lower blood glucose by a different method, blocking the digestion of carbohydrates through inhibition of enterocyte enzymes that cleave oligosaccharides to monosaccharides8. These drugs can be useful particularly if postprandial glucose absorption is a concern, although they are often associated with undesirable gastrointestinal effects.
Another approach to lowering blood glucose is through sodium-glucose co-transporter 2 (SGLT2) inhibition, which blocks reabsorption of glucose by the kidney and increases its secretion into the urine9. These inhibitors have been recently FDA-approved, are effective at decreasing hyperglycemia, and may decrease adverse cardiovascular outcomes in type 2 diabetes patients247. However, they lack long-term safety data and may also cause urinary tract infections. Finally, bile acid sequestrants which lower cholesterol by binding to bile acids, also lower blood glucose by an unknown mechanism 10. However, as such agents bind bile acids in the intestine, they are also associated with adverse gastrointestinal effects.
Existing diabetes drugs _target only a subset of the potentially druggable pathways that regulate glucose homeostasis, particularly in the liver15. As the chief producer of glucose that is released to the circulation, the liver is an especially important organ in controlling blood sugar16. Moreover, in addition to its ability to produce glucose, the liver can also store glucose as glycogen or utilize glucose as a carbohydrate fuel. Leveraging novel _targets for decreasing liver glucose production or promoting liver glucose storage may enable more efficient suppression of diabetic symptoms. Such agents would likely be used combinatorially with existing drug therapies when enhanced suppression of blood glucose levels is necessary, and may enable the use of lower concentrations of individual drugs, thereby limiting side effects.
A small number of liver-_targeted approaches, which aim to reduce gluconeogenesis or promote glycogen storage, are already being investigated in the clinic. These include glucokinase activators17–24, glycogen phosphorylase inhibitors25–34, glucagon receptor antagonists35–41, fructose 1,6-bisphosphatase inhibitors42–44, as well as protein tyrosine phosphatase 1B inhibitors45–54, with new potential _targets continuing to emerge. In this review, we will provide an overview of the physiology and molecular pathways of liver glucose homeostasis, gluconeogenesis and glycogenolysis, assessing current and novel type 2 diabetes drug _targets.
Glucose homeostasis and type 2 diabetes
Circulating blood glucose is derived from intestinal absorption from food, glucose production (or gluconeogenesis), and glycogen breakdown (or glycogenolysis)55. The liver is a major metabolic organ, and among its many functions, it is chiefly responsible for gluconeogenesis (with the kidneys contributing an average 20% to glucose release56, and the gut supplying up to 15–20%57) and glycogenolysis58. Gluconeogenesis and glycogenolysis by the liver lead to glucose release into the blood and a rise in blood glucose levels, with subsequent uptake of glucose by peripheral tissues including the kidney, skeletal muscle, splanchnic organs, and adipose tissue59 (Figure 1). Following feeding, postprandial insulin is secreted from pancreatic beta cells to directly inhibit glycogenolysis and increase glycogen synthesis60,61, and to indirectly inhibit gluconeogenesis through suppression of glucagon and inhibition of adipose free fatty acid release62. Insulin also preferentially increases uptake of glucose by peripheral tissues63. Conversely, during times of fasting, glucagon is secreted from pancreatic alpha cells to increase gluconeogenesis and glycogenolysis in the liver64. Insulin levels also fall, and peripheral tissues utilize proportionately less glucose as fuel relative to fatty acids. In the 24 hours following absorption of a meal, glycogenolysis in the liver substantially contributes to glucose release into the circulation65,66.
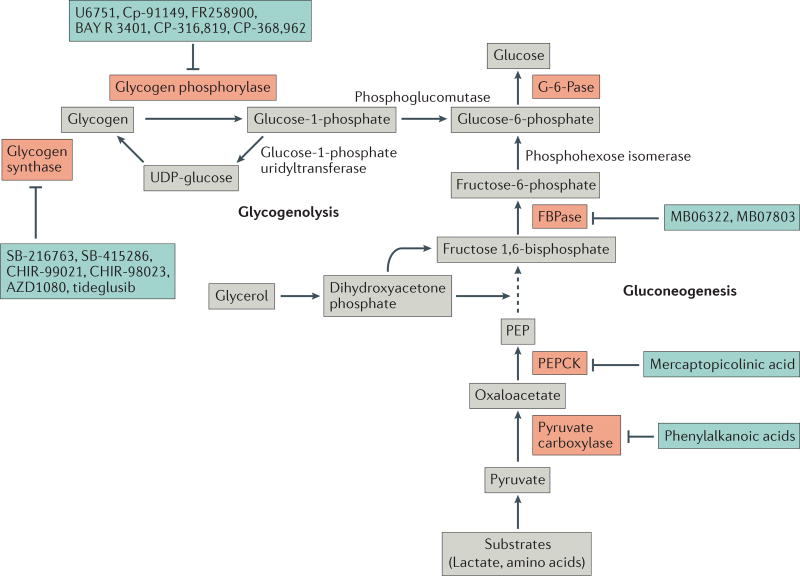
The nexus of gluconeogenesis and glycogenolysis lies at glucose-6-phosphate, which is a phosphorylated form of glucose that cannot be exported by the cell until it is dephosphorylated by glucose-6-phosphatase (G-6-Pase, Figure 2). In gluconeogenesis, the substrates lactate, amino acids, and glycerol are used by the liver to generate glucose-6-phosphate67. During glycogenolysis, glycogen is broken down and converted to glucose-6-phosphate. Glycogen, which consists of polymerized glucose, serves as a readily mobilized storage pool of carbohydrate fuel which can be phosphorylated and quickly used to generate glucose when needed.
Figure 2. Key steps of gluconeogenesis and glycogenolysis.
In gluconeogenesis, lactate and amino acids are first converted to pyruvate, either directly or indirectly through tricarboxylic acid (TCA) cycle intermediates. Pyruvate is then shuttled from the cytosol into the mitochondria where it is used to generate oxaloacetate by pyruvate carboxylase. Oxaloacetate is converted to aspartate, exported from the mitochondria and re-converted to oxaloacetate, and then is made into phosphoenolpyruvate (PEP) by PEP carboxylase (PEPCK). PEP is catalytically altered to fructose 1,6-bisphosphate, and glycerol can also enter gluconeogenesis through conversion to fructose 1,6-bisphosphate. Fructose 1,6-bisphosphate is then altered to fructose-6-phosphate by fructose 1,6-bisphosphatase (FBPase), then to glucose-6-phosphate by phosphohexose isomerase. Finally, the phosphate on glucose-6-phosphate is removed by the liver-specific enzyme glucose 6-phosphatase (G-6-Pase) to generate glucose, which can be exported to the circulation. During glycogenolysis, the glucose residues in glycogen are phosphorylated by glycogen phosphorylase to produce glucose-1-phosphate, then glucose-6-phosphate through phosphoglucomutase92. The reverse reaction is catalyzed by glycogen synthase, which generates glycogen from UDP-glucose that is converted from glucose-1-phosphate by glucose-1-phosphate uridyltransferase. As in gluconeogenesis, the glucose-6-phosphate produced through glycogenolysis is converted to glucose by G-6-Pase, and released to the circulation. Enzymes that have been investigated for drug _targeting are highlighted in red.
Normally, glucose production and uptake are kept in balance to maintain glucose homeostasis and a fasting blood glucose range of 70 to 90 mg/dL in humans68. Type 2 diabetes develops when insufficient insulin is produced to maintain this level of blood glucose, generally as a combined result of insulin resistance in peripheral tissues and beta cell dysfunction, leading to persistently elevated blood glucose, or hyperglycemia68. As diabetes is a chronic condition, its progression is characterized by advanced beta cell failure69. Additionally, aberrantly high levels of glucagon can persist in diabetic patients, leading to elevated rates of gluconeogenesis and glycogenolysis in the liver that contribute to hyperglycemia70,71. In diabetic patients, gluconeogenesis also has a proportionately larger contribution to glucose production relative to normal individuals72. Moreover, the ability of diabetic patients to store glycogen may be decreased compared to healthy individuals, which also enhances postprandial hyperglycemia73. Prolonged hyperglycemia during diabetes causes vascular dysfunction that damages major organs, including the kidney, brain, heart, blood vessels, and eyes74. Thus, treating the hyperglycemia of diabetes is of the utmost importance in these patients.
Strategies to modulate liver glucose and glycogen metabolism
Overall, an imbalance in glucose release from the liver and uptake from peripheral tissues can lead to the persistent hyperglycemia that is a major contributing factor to diabetes development. Therefore, drugs to treat type 2 diabetes can be aimed at maintaining normal blood glucose levels through inhibition of gluconeogenesis and/or glycogenolysis in the liver, or through stimulation of glucose uptake into tissues. The most commonly used type 2 diabetes therapy, metformin, is known to decrease hepatic gluconeogenesis, but the precise molecular mechanisms of this agent have not been well defined4 (see Box 1). Moreover, metformin can cause various side effects and it typically must be used in combination with other drugs in order to achieve long term suppression of hyperglycemia11,75. Other liver-_targeted agents which have been or are currently being investigated in clinical trials include: activators of glucokinase (which converts glucose to glucose-6-phosphate in the first step of glycolysis)17–23, inhibitors of fructose 1,6-bisphosphatase (which converts fructose-1,6-bisphosphate to fructose 6-phosphate in gluconeogenesis)42–44, inhibitors of protein tyrosine phosphatase 1B (which is a negative regulator of the insulin signaling pathway)45–54, inhibitors of glycogen phosphorylase (which catalyzes the rate-limiting step in glycogenolysis)25–34, as well as antagonists of the glucagon receptor35–41. However, so far, no agents in these categories have received approval. The development of novel agents aimed at _targeting gluconeogenesis and glycogen breakdown in the liver may provide effective alternative diabetic treatment options. Potential therapeutic strategies include the direct _targeting of specific metabolic enzymes of gluconeogenesis and glycogenolysis, or of regulators of these processes.
Glucokinase activation
Glucokinase (GK), an enzyme predominately expressed in the liver and pancreas which phosphorylates glucose to glucose-6-phosphate in the first step of glycolysis, may be activated to inhibit gluconeogenesis. Unfortunately, several early studies with direct allosteric GK activators, such as AZD1656 (AstraZeneca)17, were abandoned during the clinical phases of research due to a decline in efficacy of the compounds over time, as well as hypoglycemic effects18. However, several GK activators remain in clinical development18. The liver-selective GK inhibitor PF-04991532 (Pfizer) exhibited favorable glycemic effects in diabetic rats19, but newer findings indicated the presence of oxidative metabolites of the compound in human plasma20, which will require further careful examination if development is continued. More recent clinical studies employing the GK activators piragliatin (Roche) and AMG 151 (Amgen; previously Array BioPharma (ARRY-403)) demonstrated that both agents resulted in hypoglycemia, with a slight increase in circulating triglycerides for the latter21,22. The outcomes of a 6-month phase II clinical trial with a liver-selective GK activator TTP399 (Trans Tech Pharm) are pending following promising short-term effects, including glucose lowering after 6 weeks without hypoglycemia or increased plasma lipids24.
Another approach to activating GK is to inhibit its binding to GK regulatory protein (GKRP). GKRP binds cytoplasmic GK when glucose levels decrease, sequestering GK in the nucleus in its inactive form. The benefit of this approach is that liver-specific GKRP inhibition decreases blood glucose without the risk of hypoglycemia that can result from activating GK or inhibiting pancreatic GKRP76. The piperazine compound AMG-3969 dissociated GK and GKRP in rat livers, and decreased blood glucose in diabetic but not normoglycemic mice23. These findings suggest that GKRP inhibitors may represent promising candidates for clinical investigation.
PTP-1B inhibition
Insulin exerts suppressive effects on glucose production through the promotion of insulin receptor phosphorylation, which triggers downstream signaling77. Protein tyrosine phosphatase 1B (PTP-1B) dephosphorylates the insulin receptor, opposing the action of insulin. Since phosphorylation of the insulin receptor is decreased in type 2 diabetes78, PTP-1B is an attractive diabetes drug _target. Mice deficient in PTP-1B display enhanced hepatic insulin sensitivity, as indicated by an increase in phosphorylation of the insulin receptor in liver; furthermore, these mice are resistant to gaining weight on a high fat diet79. Mice with PTP-1B specifically deleted in the liver display enhanced hepatic insulin signaling, improved glucose tolerance and homeostasis, and increased suppression of hepatic glucose production in insulin-resistant and high fat diet-induced obesity models80,81. These and other studies further justify _targeting hepatic PTP-1B for diabetes treatment82. Studies in monkeys and mice have reported that ISIS 113715 (ISIS Pharmaceuticals), an antisense oligonucleotide _targeting PTP-1B that is not liver-specific, reduces fasting concentrations of glucose, while improving insulin sensitivity and decreasing hepatic triglyceride accumulation46,47. ISIS 113715 has shown promising effects in decreasing fasting blood glucose levels in combination with sulfonylureas in a phase II clinical trial involving type 2 diabetes patients45.
_targeting PTP-1B with small molecule inhibitors has also been attempted. Ertiprotafib (Wyeth Pharmaceuticals; acquired by Pfizer) showed initial promise by lowering blood glucose levels when orally administered to mice48 and progressed to a phase II clinical trial. Further studies also indicated decreased insulin levels, along with lowered triglyceride and free fatty acid levels; however, these outcomes were reported to be likely due to off-_target effects of ertiprotafib, which include activation of peroxisome proliferator-activated receptor α (PPARα) and PPARγ49, and potent inhibition of IκB kinase β (IKK-β)50. Phase II trials with ertiprotafib were discontinued, likely due to its lack of in vivo efficacy, coupled with unwanted side effects49. Trodusquemine (MSI-1436), developed by Genaera Corporation (formerly Magainin Pharmaceuticals), is a spermine analog that was initially observed to modulate body weight, glucose homeostasis and serum cholesterol levels in ob/ob mice51, and was later determined to selectively inhibit PTP-1B52. As of 2013, trodusquemine was in phase II clinical trials for treatment of obesity and diabetes53. More recently, a chemical biology approach has uncovered an allosteric mechanism of PTP-1B inhibition by trodusquemine54. Carefully designed small molecules allosterically _targeting PTP-1B could enhance specificity, which could improve in vivo efficacy and reduce unwanted off-_target effects.
Glycogen synthesis stimulation
Glycogen phosphorylase inhibition
One method to inhibit glucose release by the liver is to increase its storage as glycogen. In diabetic patients, hepatic glycogen synthesis is impaired83 and the stimulation of glycogen synthesis in skeletal muscle by insulin is stunted, contributing to insulin resistance84. Ectopic lipid accumulation in the liver and skeletal muscle leads to diminished insulin signaling and decreased hepatic glycogen synthesis85. Human liver glycogen phosphorylase inhibitors can increase glycogen synthesis by interfering with glucose-6-phosphate generation during glycogenolysis26. A major appeal of these compounds is that they inhibit the enzyme at elevated blood glucose levels, but their potency diminishes when blood glucose is lowered, decreasing the likelihood of developing hypoglycemia27. Indeed, treatment of db/db mice with the dihydropyridine diacid glycogen phosphorylase inhibitor U6751 or ob/ob mice with CP-91149 lowered blood glucose without producing hypoglycemia, but had no effect on blood glucose levels in normal mice28,29. On a molecular level, indole-2-carboxamides act as allosteric inhibitors that bind to the indole site at the dimer interface of glycogen phophorylase in order to stabilize its inactive conformation, and which also synergize with other inhibitors such as glucose30. Notably, the IC50 of a compound designed to fit into both chloroindole binding pockets of glycogen phosphorylase was only 6 nM30.
The glycogen phosphorylase inhibitor FR258900 was discovered in a screen for increased glycogen synthesis in rat primary hepatocytes86. FR258900 is a 23-carbon pentanedioic acid that binds to the AMP site of glycogen phosphorylase and stabilizes its inactive conformation87. This compound significantly decreased plasma glucose levels and increased hepatic glycogen levels in db/db and streptozocin-induced diabetic mouse models31, but long-term effects or actions in other tissues were not studied. CP-316,819, is an indole carboxamide compound32 that decreased hepatic glucose output in fasted dogs with basal or elevated glucagon levels33, although thorough analysis of potential effects on other tissues was not performed. Another commercially available glycogen phosphorylase inhibitor, BAY R 3401, which acts by a similar mechanism as indole carboxamides, also successfully decreased hepatic glucose output and plasma glucose levels in fasted dogs34.
Limited clinical studies were performed with CP-316,819, which showed lowering of peak hyperglycemia after a glucagon challenge in normal subjects25. A related compound, CP-368,962, was able to dose-dependently lower blood glucose in type 2 diabetic human subjects, but the effect was lost after 4 weeks of treatment, demonstrating a lack of durability25. However, glycogen phosphorylase has five different ligand binding sites including its catalytic and AMP-binding sites, revealing several possible methods for _targeting its enzyme activity88. Thus, although glycogen phosphorylase inhibitors may have thus far failed due to lack of durability, alternative approaches may enable the development of an inhibitor that is able to overcome this barrier.
A potential problem with _targeting glycogen phosphorylase is that it is also present in the skeletal muscle, where it is needed to support energetic deficits89. The issue of tissue selectivity could be abated by selecting an inhibitor that is more potent against the liver isoform90. However, tissue selectivity may not be a substantial confounding issue, as a study in rat gastrocnemius-plantaris-soleus muscle suggested that treatment with glycogen phosphorylase inhibitors did not deplete skeletal muscle function89. Additionally, glycogen phosphorylase inhibitors may be beneficial to cardiac muscle, where they can reduce glycolysis and proton production which are harmful during myocardial ischemia91.
Glycogen synthase activation
Glycogen synthesis can also be _targeted through activation of glycogen synthase92. Although glycogen phosphorylase inhibition can decrease inhibition of glycogen synthase93, this enzyme could also be _targeted independently. A major regulatory kinase for glycogen synthase is glycogen synthase kinase 3 (GSK-3), which phosphorylates and inactivates glycogen synthase94. GSK-3 levels and activity are increased in skeletal muscle of patients with type 2 diabetes95, and in the adipose tissue of obese diabetic mice96, suggesting that GSK-3 contributes to insulin resistance.
Two maleimide compounds, SB-216763 and SB-415286, were discovered to be GSK-3 inhibitors within the nanomolar range with considerable specificity over other kinases97. These compounds were effective in activating glycogen synthase in human liver cells97, and suppressed PEPCK and G-6-Pase in hepatoma cells98. Other specific and potent GSK-3 inhibitors have also been used in vivo99. The aminopyrimidine derivatives CHIR-99021 and CHIR-98023 significantly increased glucose disposal in Zucker diabetic rats, which was accompanied by an increase in hepatic glycogen synthesis with no change in skeletal muscle glycogen synthesis100. These inhibitors also lowered blood glucose in db/db mice without causing hypoglycemia101. In addition, the GSK-3 peptide inhibitor L803-mts decreased blood glucose and PEPCK expression, and increased hepatic glycogen in ob/ob mice102.
While the effects of GSK3 inhibitors on blood glucose are promising, hesitation to move these inhibitors to the clinic may stem from concerns over their potential activation of the oncogene β-catenin, although no studies have shown increased tumorigenicity with GSK-3 inhibitor use103. Moreover, potent GSK-3 inhibition can lead to toxicity (as occurs with lithium treatment in the brain, causing motor deficits104), so care must be taken to only mildly inhibit the enzyme. AZD1080, a GSK-3 inhibitor from AstraZeneca, exhibited some success in limited phase I clinical trials for Alzheimer’s disease, but blood glucose was not measured105. Another GSK-3 inhibitor, tideglusib (Noscria), was used in a phase II clinical trial for progressive supranuclear palsy106. Although tideglusib was generally found to be safe, it was not effective in treating progressive supranuclear palsy and the effects on blood glucose were not evaluated. Concerns were raised surrounding the irreversible nature of this inhibitor107, which may make toxicity more likely and its clinical use difficult.
Glucagon receptor antagonism
In patients with type 2 diabetes, increased secretion of glucagon from pancreatic α-cells, in addition to heightened hepatic sensitivity towards glucagon, contributes to the elevated hepatic glucose production observed in these individuals108,109. _targeting the glucagon receptor may therefore be a promising approach for decreasing hepatic glucose production and reducing fasting plasma glucose levels110. In support of this approach, two glucagon receptor peptide antagonists decreased blood glucose and improved insulin sensitivity in ob/ob and diet-induced obese mice111. In mice expressing human glucagon receptor, the glucagon antagonist compound 1 decreased blood glucose in response to glucagon administration112. In addition, NNC 25-0926, a potent glucagon antagonist with an IC50 of 12 nm, blunted the glucagon-induced increase in blood glucose in fasted dogs113. Additionally, the selective and reversible competitive glucagon receptor antagonist compound 9m (also known as MK-0893 (Merck)), lowered glucagon-induced blood glucose in rhesus monkeys, and in humanized ob/ob mice fed a high fat diet39.
Glucagon receptor antagonists have been used in several studies in humans. The orally available Bay 27-9955 (Bayer) decreased short-term fasting glucose levels without exhibiting any prominent side effects37, but was no longer pursued for clinical development for undisclosed reasons. LGD-6972 (Ligand Pharmaceuticals) similarly demonstrated a dose-dependent decrease in fasting glucose in normal and diabetic subjects and favorable safety profiles in a phase 1b clinical study, and a phase II trial is currently underway to better assess its safety and efficacy36. In addition, once daily dosing with PF-06291874 (Pfizer) decreased fasting and postprandial glucose levels in patients on metformin, with or without combined treatment with a sulfonylurea, for up to 28 days35. However, although this drug exhibited little risk of hypoglycemia, it was associated with a small increase in LDL cholesterol and aminotransferase levels. A similar increase in LDL cholesterol levels was observed in clinical trials of MK-0893, resulting in discontinuation of this agent38,39. While an initial short-term clinical study with LY2409021 (Eli Lilly) showed encouraging decreases in fasting and postprandial glucose levels, they also raised concerns due to a rise in aminotransferases40. However, a more recent longer duration phase II study indicated that doses of LY2409021 which are sufficient to lower fasting glucose levels, only modestly increased aminotransferase levels, and clinical development is ongoing41. These results suggest that glucagon antagonists may be titrated for therapeutic use with minimal side effects.
Modulating pyruvate flux
Pyruvate carboxylase inhibition
Insulin has been found to decrease hepatic glucose production by suppressing pyruvate flux through inhibition of adipose lipolysis, which decreases hepatic acetyl-CoA, a potent activator of pyruvate carboxylase114. Therefore, inhibiting pyruvate carboxylase may be a strategy for diabetes treatment. Indeed, in a study of human liver biopsies, pyruvate carboxylase levels correlated significantly with hyperglycemia115. Furthermore, inhibition of pyruvate carboxylase through antisense oligonucleotide _targeting in the liver and adipose tissue of high fat diet-fed and diabetic mice lowered blood glucose and rates of gluconeogenesis115. Additionally, adiposity and hepatic steatosis were decreased owing to reduced glycerol synthesis. Although phenylalkanoic acids can inhibit pyruvate carboxylase, and phenylproprionic acid decreased gluconeogenesis at least acutely in normal and diabetic rats116, these compounds lack tissue specificity. Additionally, phenylalkanoic acids may inhibit insulin secretion, as phenylacetic acid treatment of cultured beta cells and rat islets decreased glucose-stimulated insulin release117. Therefore, although this is a promising antidiabetic strategy, improved pharmacological or non-pharmacological approaches would need to be developed in order to selectivity inhibit this enzyme in a clinical setting.
Inhibition of mitochondrial pyruvate import
Another method to inhibit pyruvate flux into gluconeogenesis is to block pyruvate transport across the inner mitochondrial membrane into the mitochondrial matrix, where it generates oxaloacetate for use in glucose production118. A heteroligomeric complex of mitochondrial pyruvate carrier 1 (MPC1) and MPC2 is necessary and sufficient for this transport of pyruvate119,120. Independent studies assessed the role of these proteins in the control of gluconeogenesis. Liver MPC1 KO mice exhibited compensatory increased glutamine usage and urea cycle activity, which also produce oxaloacetate, preventing a decrease in basal gluconeogenesis121. However, despite this compensation, hyperglycemia was decreased and glucose tolerance was improved in the MPC1 KO mice fed a high fat diet. Similarly, knockout of hepatic MPC2 induced compensatory amino acid metabolism and decreased hyperglycemia in db/db and streptozotocin-induced models of diabetes122.
The effects of MPC1 and MPC2 on blood glucose in diabetes models suggest that _targeting of these proteins by drugs may represent a promising diabetes therapy. The established MPC inhibitor UK-5099 suppressed glucose production from primary hepatocytes121 and increased glucose uptake into myocytes123. Intriguingly, TZD compounds (Box 1) can also directly inhibit the MPC and improve glucose handling in a PPARγ-independent manner123,124. Specific _targeting of MPC in the liver may therefore improve blood glucose levels without leading to undesirable side effects due to effects on other tissues, as can occur with TZDs. In addition, such liver-specific MPC inhibition would ensure that pyruvate uptake into tissues that are critically dependent on glucose as a fuel source is not inhibited. However, the potential for compensatory effects would need to be carefully evaluated in a clinical setting, particularly with regard to the increase in glutamine metabolism, as glutamine anaplerosis and oxidation can help fuel the TCA cycle and fatty acid synthesis which drive tumorigenesis121,125.
_targeting gluconeogenesis enzymes
Inhibition of PEPCK and G-6-Pase
Two gluconeogenic enzymes with the potential to be _targeted for diabetic treatment are PEPCK, which converts oxaloacetate to PEP, and G-6-Pase, which catalyzes the conversion of glucose-6-phosphate to glucose. The expression of these enzymes are highly regulated by glucagon and insulin in correlation with gluconeogenic flux126–129. In addition, PEPCK expression is dysregulated in diabetes, and a 7-fold increase in the expression of PEPCK results in hyperglycemia in mice130. PEPCK has therefore been thought to be a rate-limiting enzyme for gluconeogenesis and has been implicated as a potential _target to reduce hepatic glucose production and blood glucose. Indeed, inhibition of PEPCK by 3-mercaptopicolinic acid results in hypoglycemia131. However, the notion that PEPCK is rate-limiting in gluconeogenesis has recently been challenged, particularly since neither PEPCK nor G-6-Pase levels are elevated in livers from patients with type 2 diabetes132. Specifically, liver-specific PEPCK knockout mice maintain normal blood glucose levels even after 24 hours of fasting133,134, due to increased extrahepatic gluconeogenesis and reduced whole-body glucose turnover134. In addition, the amount of PEPCK in the liver exhibits poor control strength on hepatic glucose production, but very strong control of flux through the TCA cycle, the inhibition of which ultimately leads to triglyceride accumulation in liver133,135,136. These studies highlight the importance of PEPCK in cataplerosis (i.e. the removal of TCA cycle intermediates), and suggest that it might also support the energetic demands of gluconeogenesis via maintenance of TCA cycle flux. Although the control strength of PEPCK on gluconeogenic flux is less than is expected of a rate-limiting enzyme, small changes in PEPCK can still lead to decreased gluconeogenic flux136.
Interestingly, mice lacking G-6-Pase in the liver also maintain normal blood glucose levels during prolonged fasting137. As noted for PEPCK knockout mice, this is due to increased levels of extrahepatic gluconeogenesis137. In addition, G-6-Pase knockout mice are protected from diet-induced obesity, due to increased energy expenditure in peripheral tissues138.
The studies with PEPCK and G6Pase genetic models imply that complete inhibition of one of these enzymes singularly in the liver might not be sufficient to reduce blood glucose in vivo in the nondiabetic state. However, these studies do not preclude the possibility that decreases in the activity of these genes may improve hyperglycemia, particularly in the diabetic state. To this end, partial silencing of PEPCK by RNAi decreased blood glucose levels and free fatty acids in diabetic mice while avoiding hepatic steatosis and lactic acidosis104. Moreover, PEPCK and G-6-Pase are part of a coordinated response to the fasted state, which involves many cellular signaling changes including upregulation of gluconeogenic pathways, a switch from glucose to fatty acid oxidation, and increased autophagy, among others139,140. Therefore, _targeting this response (for example, by _targeting post-translational modifications of PGC-1α – see below), rather than direct inhibition of either enzyme alone, might prove to be a more effective strategy to control hyperglycemia. _targeting PEPCK and G-6-Pase indirectly through modulation of its regulators may also allow for additional metabolic alterations, including redirection of carbon intermediates, which can circumvent potential problems of _targeting these enzymes directly.
FBPase inhibitors
FBPase, which converts fructose-1,6-bisphosphate to fructose-6-phosphate, is another gluconeogenic enzyme with the potential to be inhibited for diabetes treatment. Treatment of diabetic rats with the direct FBPase inhibitor and phosphanate prodrug MB06322 (Metabasis Therapeutics, also known as CS-917) led to decreased hyperglycemia, without causing aberrant metabolic alterations42. This inhibitor was an 8,9-disubstituted purine which acts as an AMP mimetic to bind to the allosteric site of FBPase. Benzimidazole analogs with IC50 values of less than 100 nM were developed which lowered glucose in rats and inhibited human liver FBPase43. Further steps have been taken to determine whether this class of compounds could be developed for the clinic. Specifically, MB07803 is a second generation FBPase inhibitor based on CS-917 which was used in a phase Ib clinical trial in type 2 diabetes patients44. In this short-term study, blood glucose levels were lowered in the 6 hours following a 12 hour fast. However, some patients experienced nausea and vomiting at higher doses. Additional studies will need to be conducted regarding the long term effects of this drug class, as well as the potential development of an FBPase inhibitor that does not induce gastrointestinal side effects.
Activation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase
A potent regulator of glycolytic and gluconeogenic flux is fructose-2,6-bisphosphate (F-2,6-P2), which is a product of the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (6PFK2/FBP2). Insulin stimulates the kinase activity of this enzyme, resulting in increased levels of F-2,6-P2 that allosterically activates phosphofructokinase-1 (PFK-1), the committed step in glycolysis which has the opposite effect of FBPase and converts fructose-6-phosphate to fructose 1,6-bisphosphate141. In contrast, glucagon stimulates the BPase activity of the enzyme, resulting in reduced levels of F-2,6-P2 and elevated gluconeogenesis141. In accordance to its importance in controlling glucose flux, increasing the levels of F-2,6-P2 in the liver improves insulin sensitivity and lowers blood glucose in mice142, making the bifunctional enzyme a potential _target to reduce hepatic glucose production. In addition, overexpression of the bifunctional enzyme in mouse liver lowers blood glucose through suppression of hepatic glucose production143, further supporting the concept that activation of this enzyme by small molecules might have beneficial effects on glucose homeostasis.
Modulating gluconeogenic transcription factor and coactivator activity
_targeting transcription factors and coactivators could be a potentially effective method for developing treatments for type 2 diabetes, as these regulators can affect entire pathways and responses that are physiologically regulated by fasting and feeding. However, transcription factors are frequently found in multi-protein complexes, and designing small molecules that will potently change complex activity can be difficult144. Various confounding factors include the large molecular area involved in protein-protein interactions, which may not contain ideal pockets for small molecules to bind; the large and flexible structure of coactivators which can be accompanied by an unknown crystal structure; a lack of ligand-binding sites; and limited small molecule libraries with properties ideal for _targeting transcription145,146. However, small molecules may allosterically affect protein-protein interactions to ultimately affect multi-protein complex activity either by disrupting the integrity of the complex or by changing post-translational modifications (PTMs) of specific subunits147. Indeed, successful approaches have been employed to _target transcriptional proteins, including the identification of a direct inhibitor of steroid receptor coactivator 3 (Src-3) through high-throughput screening of small molecules that alter Src-3-dependent transcription145. Moreover, modulating transcription factor activity – by intervening with the interaction of transcription factors with their cofactors - has been a major focus in drug development for the treatment of cancer148,149.
PGC-1a
Several regulators of liver metabolic transcription have the potential for diabetes drug _targeting. PPARγ coactivator-1α (PGC-1α) is a transcriptional coactivator that is activated in response to nutrient signals150. Initially, PGC-1α was identified in brown adipose tissue as a cold-inducible coactivator of nuclear receptors151, with high expression observed in other metabolic tissues151,152. PGC-1α is a chief regulator of mitochondrial biogenesis and function, and consistent with its cold-inducible status, is an activator of mitochondrial uncoupling153.
In the liver, PGC-1α expression is modulated acutely by nutritional status, as it acts as a primary inducer of gluconeogenesis154. In the fed state, hepatic PGC-1α levels are low, and gluconeogenesis is suppressed154. Conversely, under fasting conditions, PGC-1α levels are markedly enhanced to stimulate gluconeogenesis and fatty acid oxidative metabolism154. The induction of PGC-1α under fasting is achieved through the action of glucagon155 (Figure 3a). When induced, PGC-1α coactivates the glucocorticoid receptor (GR), hepatic nuclear factor 4α (HNF4α), and forkhead box O1 (FoxO1), leading to PEPCK and G-6-Pase transcription139,154,155. In addition, PGC-1α increases hepatic insulin resistance and fatty acid oxidation through coactivation and induction of PPARα156. Insulin resistance is increased through PPARα-dependent upregulation of Tribbles homolog 3 (Trb3), a negative regulator of Akt, while fatty acid oxidation is enhanced by PPARα-driven transcription of fatty acid oxidation genes.156 This increase in fatty acid oxidation provides carbon substrates to the TCA cycle, which in turn contributes to increased gluconeogenic flux.
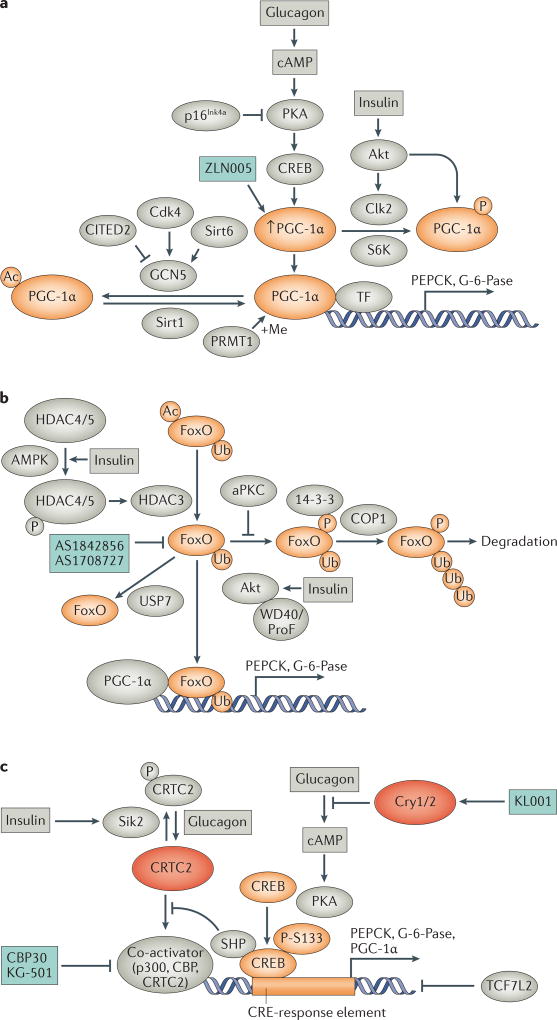
Figure 3. Regulation of gluconeogenic transcription factor and coactivator activity.
a. Regulation of PGC-1α. During fasting, induction of PGC-1α in the liver is achieved through the action of glucagon, which increases cyclic AMP (cAMP), PKA activity, and CREB to increase PGC-1α transcription155. Enhanced PGC-1α expression is inhibited by the Cdk inhibitor p16Ink4a, which decreases gluconeogenic gene expression through suppression of PKA activity252. Activated PGC-1α coactivates transcription of several transcription factor (TF) binding partners, including HNF4, FoxO1, and GR, to increase transcription of _target genes such as PEPCK and G-6-Pase. PGC-1α activity can be altered by PTMs, including acetylation and phosphorylation which decrease its activity, and methylation which increases its activity. Insulin decreases PGC-1α activity by increasing its phosphorylation by Akt and Clk2, and S6K also inhibits PGC-1α through phosphorylation. PGC-1α acetylation is decreased by Sirt1 and increased by GCN5, whose activity is increased by Cdk4 and Sirt1 and inhibited by CITED2. PGC-1α is methylated by PRMT1.
b. Regulation of FoxO activity. The transcription factor FoxO is co-activated by PGC-1α to increase transcription of gluconeogenic genes. FoxO activity is modulated by PTMs, including phosphorylation by Akt which leads to its polyubiquitination and degradation, and removal of monoubiquitination by USP7 which decreases its transcriptional activity. FoxO can also be activated through deacetylation by HDAC3 in response to insulin signaling.
c. Regulation of CREB activity. CREB is a transcription factor that increases gluconeogenic gene expression from CRE-response elements when activated by a co-activator such as p300, CBP, or CRTC2. CREB activity is increased by phosphorylation at S133, which is enhanced by glucagon-stimulated PKA activity, which is inhibited by Cry1/2. CRE-response element transcription can also be increased by glucagon through dephosphorylation of CRTC2, which increases its activity as a CREB co-activator, or inhibited by insulin-dependent Sik2-mediated phosphorylation of CRTC2, decreasing its activity as a co-activator. CREB activity is inhibited by binding of the transcription factor TCF7L2 to CRE-response elements, which blocks binding of CREB.
Potential therapeutic strategies and agents _targeting the activity of PGC-1α, FOXO and CREB are shown.
P, phosphorylation; Ac, acetylation; Me, methylation; Ub, ubiquitination.
Several studies have investigated the role of PGC-1α in regulating blood glucose levels in knockout or knockdown mouse models. While one study found no change in gluconeogenesis or PEPCK and G-6-Pase gene expression in liver-specific knockout mice157, others determined that fasting hypoglycemia occurs with homozygous knockout, heterozygous knockout, and/or with RNAi-mediated PGC-1α knockdown156,158,159. Another study found that PGC-1α knockout specifically decreases glucose production due to reduced gluconeogenic flux from PEP, secondary to reduced fatty acid oxidation and TCA cycle flux160. The discrepancy likely results from differences in the mouse models and strains used, but the majority of the evidence is in support of PGC-1α as a regulator of blood glucose levels. Complete and chronic ablation of PGC-1α may result in activation of compensatory mechanisms that mask the effect of PGC-1α inactivation; however, suppressing PGC-1α in the liver has the potential to reduce glucose production.
In addition its transcriptional regulation, PGC-1α activity is also regulated by PTMs, including phosphorylation161,162, acetylation163–167, and methylation168 (Figure 3a). Insulin suppresses PGC-1α activity by increasing its phosphorylation at serine 570 by Akt161, and through Akt-induced Cdc2-like kinase 2 (Clk2), which phosphorylates the PGC-1α serine-arginine (SR) domain162. SR domain phosphorylation is also increased by insulin-induced S6 kinase (S6K), enabling decreased gluconeogenic activity of PGC-1α specifically by interfering with its interaction with HNF4169. PGC-1α activity has been found to be increased by deacetylation and decreased by acetylation163. The deacetylation of PGC-1α is regulated by fasting and feeding, as the activity of the deacetylase sirtuin 1 (Sirt1) and its interaction with PGC-1α are enhanced during fasting164. Additionally, Sirt6 has been found to deacetylate general control of amino acid synthesis protein 5 (GCN5), increasing its acetyltransferase activity toward PGC-1α165,166. PGC-1α can also be acetylated through cyclin D1-Cdk4, whose activity is increased by feeding and in db/db mice, and which activates GCN5167. A protein discovered to deacetylate PGC-1α, named CBP- and p300-interacting transactivator with glutamic acid- and aspartic acid-rich COOH-terminal domain 2 (CITED2), is a glucagon-stimulated inhibitor of GCN5, and knockdown of hepatic CITED2 decreased gluconeogenesis in mice170. Additionally, PGC-1α activity can be modified through methylation, with protein arginine methyltransferase 1 (PRMT1) stimulating the activity of PGC-1α168. The regulation of PGC-1α methylation during fasting and feeding has not yet been determined.
Due to its robust effect on gluconeogenesis, drug _targeting of PGC-1α in the liver is a potentially appealing strategy for the treatment of type 2 diabetes. The most significant challenge with _targeting PGC-1α is achieving selective inhibition of its gluconeogenic function in the liver, without inhibiting its effect on mitochondrial function in the liver and other metabolic tissues. However, potential strategies to possibly achieve this goal are multifold. One such strategy is to _target specific PTMs of PGC-1α that only affect its activity toward gluconeogenic _targets, perhaps by _targeting the interaction between PGC-1α and HNF4α or FoxO1. This strategy is supported by the finding that S6K can specifically _target the interaction of PGC-1α with HNF4α169. Although manipulating specific protein-protein interactions or PTMs within a multi-protein complex is not a simple task, lessons learned from the regulation of other transcriptional complexes, such as the NF-ΚB pathway, suggest that it may be achievable171.
Another strategy is to alter PGC-1α stability or activity by _targeting upstream regulators that are expressed relatively specifically in the liver. Utilizing existing inhibitors of PGC-1α activators such as Sirt1 and AMPK is of limited usefulness for decreasing gluconeogenesis, as these proteins have a broad arsenal of _targets in addition to PGC-1α172,173. Furthermore, global inhibition of Sirt1 and/or AMPK would be detrimental, as these proteins have been found to beneficially alter metabolism to improve cardiac and skeletal muscle function, aging, and brain function, among other effects174,175. However, other PGC-1α regulators discussed above have the potential to be _targeted in order to alter hepatic gluconeogenesis, without aberrantly affecting other metabolic pathways. Additionally, several compounds were found to increase PGC-1α expression through high-throughput screening in skeletal muscle cells, suggesting that chemical modifiers of PGC-1α expression could also be probed in the liver176.
A small molecule named ZLN005 was discovered in a screen for compounds that increased PGC-1α transcription in HEK293 cells, and was confirmed to activate its transcription in L6 myotubes177. When used in vivo in db/db mice, ZLN005 activated transcription in skeletal muscle as expected, but inhibited PGC-1α transcription in the liver. The expression of G-6-Pase and PEPCK were decreased, while pyruvate tolerance was improved. There was also no effect on mitochondrial gene _targets in the liver. The mechanism of action of this drug is unclear, as it had no cell autonomous effect on primary hepatocytes. However, these results suggest that it may be possible to therapeutically _target PGC-1α in specific tissues, with desired inhibitory effects in liver but stimulatory effects in skeletal muscle or adipose tissue. Further understanding of the precise mechanisms regulating PGC-1α activity will aid in the development of potential liver-specific therapeutics.
FoxO proteins
As indicated above, FoxO1 is a transcription factor that is coactivated by PGC-1α in response to insulin in order to regulate hepatic gluconeogenesis155. Mammals express four evolutionally conserved FoxO proteins, named FoxO1, FoxO3a, FoxO4, and FoxO6178. While the role of FoxO1 in gluconeogenesis is well-established155, there is evidence that other FoxO proteins also contribute to this process. Mice with triple ablation of FoxO1, FoxO3, and FoxO4 have increased fasting hypoglycemia and insulin sensitivity compared to mice with FoxO1 knockout alone, suggesting that FoxO3 and FoxO4 contribute to the control of gluconeogenesis179. Additionally, hepatic glucose output is increased in FoxO6 transgenic mice, while depletion of hepatic FoxO6 causes fasting hypoglycemia, demonstrating that FoxO6 also takes part in regulating gluconeogenesis180. In addition to controlling gluconeogenesis, FoxO proteins have also been shown to regulate hepatic lipid metabolism181. FoxO1 knockout alone does not alter lipid metabolism182, but hepatic steatosis and lipid secretion are increased in FoxO1 and FoxO3 double knockout mice182, while FoxO1, FoxO3, and FoxO4 knockout mice exhibit hepatic lipid accumulation183.
FoxO activity is distinctly controlled by PTMs, which has implications for hepatic glucose output. In particular, FoxO1, FoxO3a and FoxO4 are phosphorylated by Akt in response to insulin at three consensus sites184 (Figure 3b). Phosphorylation at these sites enables binding by the 14-3-3 protein and subsequent FoxO polyubiquitination and degradation. This phosphorylation by Akt is also dependent on binding to the scaffold protein WD40/ProF, and this interaction is inhibited by atypical Protein Kinase C (aPKC)185. Ubiquitination of FoxO1 can also be modulated by ubiquitin-specific protease 7 (USP7)186, which decreases monoubiquitination of FoxO1 to deplete its transcriptional activity, and COP1, which acts as an E3 ubiquitin ligase to polyubiquitinate FoxO1 and increase its degradation187. Moreover, FoxO proteins are regulated by acetylation, whereby histone deacetylases (HDACs) 4 and 5 are phosphorylated by AMPK in response to insulin, leading to recruitment of HDAC3 and deacetylation and activation of FoxO transcription188.
_targeting of FoxO proteins, either dependent on or independent of PGC-1α activity, may be a potential avenue for developing drugs for type 2 diabetes. Indeed, a direct FoxO1 inhibitor, named AS1842856, was discovered to bind and block FoxO1 transactivation, and was successful in decreasing fasting blood glucose in db/db mice without affecting wild type mice189. Another compound found to inhibit FoxO1, named AS1708727, similarly decreased blood glucose and triglyceride levels when administered for four days to db/db mice190. Further investigation into the precise mechanism of action of these or similar compounds may yield insights into how to specifically _target FoxO proteins in the liver. Additional studies regarding the nature of PTMs of FoxOs will likely help in this endeavor. The design and development of drugs that would _target multiple FoxOs would also need to consider the potential hepatic steatosis that can result from inhibition of FoxO proteins182,183.
CREB
cAMP response element binding protein (CREB) is a transcription factor that binds to CRE-response elements and alters gene transcription in various tissues, with known effects on neuronal plasticity, memory, inflammation, and hepatic gluconeogenesis191. In the liver, glucagon activates PKA through cAMP to phosphorylate CREB at serine 133192, which enables it to interact with the coactivators p300 and CREB-binding protein (CBP)193 (Figure 3c). Glucagon also stimulates dephosphorylation of CREB-regulated transcription coactivator 2 (CRTC2, also known as TORC2), which then coactivates CREB on gluconeogenic genes194. The involvement of CREB in controlling glycemia was demonstrated in CREB knockout mice, which had fasting hypoglycemia195. PGC-1α was also found to be a direct transcriptional _target of CREB, and its expression is increased with chronic CREB activation during prolonged fasting195. In addition to its effects on gluconeogenesis and fatty acid oxidation through PGC-1α, CREB also increases the flux of fatty acids towards the TCA cycle by decreasing lipogenesis through inhibition of PPARγ196.
Several other proteins have been shown to modulate CREB activity. Small heterodimer partner (SHP) is a transcriptional repressor that binds to CREB to inhibit its interaction with CRTC2197. Insulin also inhibits CRTC2 through salt inducible kinase 2 (Sik2)-mediated phosphorylation, leading to CRTC2 degradation and reduced CREB-mediated transcription198. TCF7L2 is a transcription factor that binds to CRE to block their occupation by CREB199. Circadian proteins have also been recognized in the regulatory control of CREB and gluconeogenesis. Specifically, cryptochrome 1 and 2 (Cry1 and 2) decrease the gluconeogenic gene program by blocking glucagon-induced increases in cAMP and PKA-mediated phosphorylation of CREB200.
As CREB exerts effects on other tissues, particularly the brain, and affects PGC-1α, it is important to specifically _target CREB in the liver. _targeting coactivators or inhibitors of CREB, or the interaction of CREB with these proteins, is likely a more feasible approach than directly inhibiting CREB activity. In this regard, a bromodomain inhibitor named CBP30 has been developed which selectively _targets CBP and P300201 and exerts anti-inflammatory effects. Additionally, the protein-protein interaction between CREB and CBP was successfully inhibited by the small molecule KG-501 (2-naphthol-AS-E-phosphate), which decreased cAMP-responsive gene induction202. The potential of these agents to decrease gluconeogenesis could be explored.
Although CRTC2 has not been pharmacologically _targeted, CRTC2 knockout mice exhibited decreased hepatic glucose production with improved insulin sensitivity, and without apparent neurological deficits203. In addition, selective drug _targeting of Cry proteins has been achieved by the carbazole derivative KL001, which interacts with Cry proteins and prevents their ubiquitin-dependent degradation204. This compound effectively decreased gluconeogenic gene expression and glucose production after glucagon treatment in primary hepatocytes, although it was not utilized in a mouse model.
Together, these observations suggest that it may be feasible to _target the CREB pathway, through its coactivators or inhibitors, in order to selectively _target gluconeogenesis in the liver.
C/EBPα and β
CCAAT/enhancer-binding protein alpha (C/EBPα) is a transcription factor that increases gluconeogenic gene expression, and the loss of which results in neonatal death within 8 hours of birth due to hypoglycemia205. This regulation of gluconeogenesis is PGC-1α-independent, but dependent on particular C/EBPα residues to control subsets of _target genes206. Specifically, three consensus residues for glycogen synthase kinase 3 (GSK3) phosphorylation (T222, T226, and S230) were shown to be insulin-responsive, and knock-in mouse models were generated in which these residues were mutated to alanines206. These knock-in mice exhibited elevated hepatic expression of PEPCK and G-6-Pase in conjunction with glucose intolerance, demonstrating that these residues are responsible for regulating gluconeogenic genes and glucose tolerance without affecting lipid metabolism. Another study demonstrated that phosphorylation of C/EBPα at S21 by p38 MAPK led to increased PEPCK expression in hepatoma cells207, suggesting that liver-specific inhibition of this phosphorylation site may decrease gluconeogenesis. The related family member C/EBPβ also modulates gluconeogenic gene expression, although it is not necessary for basal regulation208. Instead, C/EBPβ decreases hyperglycemia and gluconeogenesis in streptozotocin-induced diabetes, with decreases in expression of gluconeogenic genes208.
The partial redundancy in action of C/EBPα and β makes them potentially appealing drug _targets for type 2 diabetes, particularly C/EBPβ which has not been found to affect basal gluconeogenesis, but instead partially reverses hyperglycemia208. Therefore, theoretically, a drug that specifically inhibits C/EBPβ may be able to reduce blood glucose levels without causing hypoglycemia. Additionally, drug _targeting of specific phosphorylation sites on C/EBPα may have the ability to fine-tune the control of gluconeogenesis by C/EBPα without affecting other metabolic pathways, as modulation of GSK3 phosphorylation sites affects gluconeogenic gene expression without altering lipid metabolism206. Although technically challenging, identifying small molecules that inhibit specific phosphorylation sites of _target proteins has been accomplished for other pathways through screening methods including phosphopeptide binding to fusion proteins209.
REV-ERBs and RORs
REV-ERBs and retinoic acid receptor-related orphan receptors (RORs) are nuclear receptors involved in several cellular processes, notably metabolism and circadian rhythm regulation210. REV-ERBs constitutively repress transcription at ROR response elements through binding of co-repressors. In response to binding by heme, REV-ERBα suppresses gluconeogenic genes and glucose production in HepG2 cells through increased recruitment of the nuclear receptor corepressor–histone deacetylase 3 (NCoR-HDAC3) complex211. The ROR family, which includes RORα, β, and γ, activates transcription at ROR response elements through recruitment of co-activators such as PGC-1α212 and steroid receptor co-activator 2 (SRC2)213. Loss of RORα leads to fasting hypoglycemia and decreased expression of gluconeogenic genes in the liver214.
Manipulation of REV-ERBs and RORs may be of therapeutic benefit for type 2 diabetes. Activation of REV-ERBs with agonists or inhibition of RORα with inverse agonists has the potential to inhibit hepatic gluconeogenesis215. The REV-ERB agonist GSK4112, a tertiary amine, was found to decrease gluconeogenic genes and glucose output in hepatocytes, but has been reported to lack plasma exposure216. Two additional compounds, SR9011 and SR9009, were discovered through high-throughput screening as synthetic activators of REV-ERB repressor activity217. Administration of these drugs to wild type mice for 6 days significantly altered metabolic gene expression in the liver. Although gluconeogenic gene expression was not measured in this study, hepatic PGC-1α mRNA expression was repressed, suggesting gluconeogenesis inhibition. Moreover, treatment of diet-induced obese mice with SR9009 caused weight loss, with lowered plasma glucose and lipids. The selective RORα inverse agonist and benzenesulfonamide SR3335 suppressed gluconeogenic gene expression in HepG2 cells, and decreased glucose production and plasma glucose levels in a mouse model of diet-induced obesity218.
Src1 and 2
Src-1 and −2 are members of the p160 family of coactivators that control transcription of metabolic gene networks219. Src-1 is needed to induce the gluconeogenic program during the transition from the fed to fasting state, and Src-1 KO mice develop hypoglycemia without exhibiting decreases in insulin secretion or sensitivity220. The effect on gluconeogenesis is dependent on increased expression and transcriptional activity of C/EBPα220. Src-2 also regulates gluconeogenesis, specifically through coactivation of RORα leading to increased expression of G-6-Pase213. Emphasizing the importance of Src-2 in controlling liver glucose metabolism, knockout of Src-2 in mice leads to a Von Gierke’s disease phenotype, characterized by fasting hypoglycemia and increased glycogen storage213.
Drug _targeting of Src-1 and Src-2 may be feasible, as the drug gossypol was discovered to specifically inhibit Src-1 and Src-3, resulting in cancer cell toxicity221. Additionally, a direct inhibitor of Src-3 has been identified145. However, potent inhibition of these coactivators would be undesirable due to the development of fasting hypoglycemia, and the potential toxicity to non-cancer cell types is unknown. Instead, a drug exhibiting partial and specific inhibition of Src1 and/or Src2 may be beneficial for treating type 2 diabetes. Such inhibition may potentially be accomplished by _targeting PTMs of Src-1 and Src-2, as the stability, intracellular localization, and transcription factor specificity and activation of these proteins has been found to be regulated by PTMs including phosphorylation, ubiquitination, acetylation, and methylation219. This approach may enable fine-tuning of the activity of these proteins to decrease blood glucose levels to desirable levels, without inducing hypoglycemia or other toxic side effects.
Mitochondrial uncoupling
Another strategy for _targeting hepatic glucose output is to enhance glucose utilization in the liver by altering mitochondrial uncoupling. Uncoupling of the mitochondria diffuses the proton gradient across the inner mitochondrial membrane that is used to drive ATP synthesis, leading to the release of heat and the increased consumption of metabolic substrates used by the TCA cycle to fuel oxidative phosphorylation222. Both fatty acid and glucose oxidation are thus increased by mitochondrial uncoupling. Although the mitochondrial protonophore 2,4-dinitrophenol (DNP) has been known for many years to cause weight loss, it was found to be toxic and even lethal when used clinically due to hyperthermia223. To address this, a DNP derivative (DNP-methyl ester, or DNP-ME) that can only be metabolized by the liver was developed, and its administration to diabetic rats decreased fasting plasma glucose, triglycerides, insulin, and glucose production, while improving glucose tolerance224. The direct effects of DNP-ME on the liver also indirectly reduced muscle fat content and improved muscle insulin sensitivity224. Furthermore, a controlled-release orally available version of DNP - controlled-release mitochondrial protonophore (CRMP) - was developed, which induces mild hepatic uncoupling over a much longer time-frame225. Administration of CRMP to high fat diet-fed wild type or Zucker Diabetic Fatty rats lowered plasma glucose, triglycerides, insulin, and glucose production, while improving glucose tolerance. These compounds highlight the potential of utilizing mild oral hepatic uncouplers as an antidiabetic therapeutic strategy to increase substrate oxidation by the liver to decrease gluconeogenesis and blood glucose levels.
Considerations and challenges in _targeting liver glucose homeostasis
It is generally accepted that increased hepatic glucose production, due to enhanced gluconeogenesis, is the major contributor to the increased blood glucose levels observed in diabetic patients71,226–229. Directly _targeting glucose production or storage may therefore represent an effective approach to regulating blood glucose levels. Inhibiting gluconeogenic and glycogenolytic enzymes would provide the most direct route of decreasing glucose production by the liver, and indeed there are drugs under investigation that _target these enzymes (Table 1). Metformin, the first-line drug for the treatment of diabetes (despite its disputable mechanism of action), is believed to reduce blood glucose by decreasing gluconeogenesis and hepatic glucose production4, further supporting the notion that _targeting liver glucose metabolism is a useful approach to control hyperglycemia. Novel diabetes drugs _targeting glucose homeostasis in the liver would most likely be administered in combination with existing type 2 diabetes drugs, such as metformin and SGLT2 inhibitors, to achieve sustained normal blood glucose levels, and to allow for the use of lower drug concentrations in order to avoid side effects (Box 2).
Table 1.
Pharmacological compounds _targeting liver glucose metabolism that have been shown to inhibit hepatic glucose production in vivo.
| Drug | _target | In vivo and clinical effects | Potential Limitations | Refs. |
|---|---|---|---|---|
| Metformin | Unclear, involves complex I and mGPD | Extensively used in the clinic to lower blood glucose | Gastrointestinal side effects, precise _target unknown, long-term durability as monotherapy lacking | 4, 11, 75 |
| GK activators, GKRP inhibitors | GK, GKRP | Reduced blood glucose in diabetic rat and mouse models, early clinical trials demonstrated hyperglycemia normalization | Hypoglycemia and potential toxic metabolites with GK activators, unclear durability | 17–24 |
| PTP-1B antisense oligonucleotides | PTP-1B | Decreased fasting glucose in monkey and mouse models, decreased fasting glucose in phase II clinical trial with sulfonylureas | Chronic effects unknown, no small molecule inhibitor available | 45–47 |
| PTP-1B small molecule inhibitors (ertiprotafib, trodusquemine) | PTP-1B | Lowered blood glucose in phase II trials | Sub-optimal efficacy, off-_target effects on PPARα, PPARY, IKK-β | 48--53 |
| Glycogen phosphorylase inhibitors (U6751, CP-91149, FR258900, CP-316,819, CP-368,962) | Glycogen phosphorylase | Reduction of blood glucose in db/db and ob/ob mice treated with U6571, CP-91149, or FR258900; decreased hepatic glucose output in fasted dogs treated with CP-316,819; lowered peak hyperglycemia in clinical trial of CP-316,819; short-term blood glucose lowering in clinical trial of CP-368,962 | Chronic and extrahepatic effects not well-characterized, ability to lower blood glucose chronically in the clinic is unproven | 25, 28–29, 31–34 |
| GSK-3 inhibitors (CHIR-99021, CHIR-98023, L803-mts) | GSK-3 | Decreased blood glucose in db/db and ob/ob mice | Possible inhibition of oncogene β-catenin | 99–102 |
| Glucagon receptor antagonists (Bay 27–9955, LGD-6972, MK-0893, PF-06291874, LY2409021) | Glucagon receptor | Decreased fasting and postprandial glucose levels in phase Ib and II clinical trials | Elevated LDL cholesterol, increased aminotransferases | 35–41, 112–113 |
| Phenylproprionic acid | Pyruvate carboxylase | Acute inhibition of gluconeogenesis in normal and diabetic rats | No tissue specificity, may inhibit insulin release, chronic effects unknown | 116 |
| FBPase inhibitors (MB06322 and MB07803) | FBPase | Acute and chronic decreased hyperglycemia in diabetic rats treated with MB06322; lowered blood glucose for 6 hours in clinical trial of MB07803 | Ability to inhibit FBPase chronically in the clinic is unknown, may cause gastrointestinal side effects | 42–44 |
| FoxO1 inhibitors (AS1842856, AS1708727) | FoxO1 | Decreased blood glucose and triglycerides in db/db mice | No tissue specificity, chronic effects unknown | 189–190 |
| SR3335 | RORα | Decreased plasma glucose in high fat-diet fed mice | No tissue specificity, chronic effects unknown | 218 |
| DNP-ME and CRMP | Mitochondrial uncoupling | Lowered blood glucose and triglycerides in high fat diet-fed and diabetic rats | Possible toxicity from mitochondrial uncoupling | 224–225 |
Box 2: Combination strategies to lower blood glucose.
Combining novel and existing diabetes therapies, _targeting different mechanisms of glucose homeostasis control, could lead to enhanced effects on blood glucose. Indeed, combinations of existing diabetes drugs are already used in the clinic to achieve blood glucose level goals. Often, metformin is used first; however, in most patients, glycemic control declines over time, making combination therapy an essential approach75. Metformin is usually combined with one or two drugs from different classes including insulin, sulfonylureas, TZDs, SGLT2 inhibitors, DPP-4 inhibitors, and GLP-1 agonists248,249. Clinically, considerations for what combinations to use involve assessment of efficacy, side effects, contraindications, cost, the complexity of dosing if multiple daily injections are necessary, and patient aversion to using injectable medications, as is needed for insulin and GLP-1 agonists250.
Novel diabetes drugs _targeting glucose homeostasis in the liver would most likely be used in combination with existing type 2 diabetes drugs, such as metformin, to allow the use of lower drug concentrations to avoid side effects, or to prolong the anti-diabetic effect. However, as metformin also _targets glucose homeostasis in the liver, such novel agents may be particularly beneficial in decreasing hyperglycemia when used in combination with drugs that _target other mechanisms.
Combining novel drugs _targeting hepatic glucose production with insulin sensitizers, like TZDs, which cannot result in uncontrolled insulin secretion, may impose lower risks of hypoglycemia compared with insulin secretagogues such as GLP-1 agonists. However, water retention, heart failure, and weight gain are all known possible side effects of TZDs, which must be carefully considered. While increased lactic acidosis is a potential concern when inhibiting hepatic glucose output, combination with TZDs, which increase the activity of pyruvate dehydrogenase, can ameliorate lactic acidosis by increasing lactate entry into the TCA cycle251. This highlights an additional beneficial outcome of combination therapy, which is a more balanced effect on total energy metabolism that might improve overall glycemic control outcomes. SGLT2 inhibitors that reduce glucose reabsorption by the kidney and increase its secretion, like TZDs, do not result in increased insulin secretion, and thus also impose a lower risk of hypoglycemia. Combined treatment with SGLT2 inhibitors may therefore efficiently complement the action of drugs _targeting hepatic glucose output.
Modulating glucose homeostasis in the liver presents a myriad of challenges and potential advantages when considering novel treatments for type 2 diabetes. Decreasing hepatic glucose production could strongly inhibit blood glucose levels, but hypoglycemia must be avoided, and designing a drug that specifically _targets the liver without affecting other tissues can be challenging. Therefore, potency and specificity are two key concerns when developing these drugs for in vivo administration. The potency of drugs that _target hepatic metabolic enzymes must be carefully considered, as hypoglycemia or aberrant perturbations in liver fat metabolism can result from high levels of enzyme inhibition, as occurs with PEPCK and G-6-Pase knockout134,230,231. Potent _targeting of these enzymes in extrahepatic tissues can also result in metabolic dysfunction in other tissues, as occurs in kidney-specific G-6-Pase KO mice which have renal lipid accumulation and glycogen overload232. One potential avenue to avoid hypoglycemia is to _target proteins that decrease hyperglycemia without affecting basal blood glucose levels, such as glycogen phosphorylase or C/EBPβ27,208. _targeting enzymes that are expressed predominately in the liver, such as G-6-Pase, is one obvious approach to increasing specificity233.
Another strategy to increase tissue specificity would be to modify drugs so that they can be metabolized only by the liver, as was performed in the development of the DNP derivative discussed above224. The development of this liver-specific DNP mitochondrial uncoupler also revealed a more indirect method of decreasing glucose production in the liver by increasing hepatic metabolic substrate oxidation. This drug _targets the liver specifically, but also has beneficial extrahepatic secondary effects such as decreased fat and improved insulin sensitivity of muscle. Titrating a therapeutic to generate mild uncoupling without inducing toxicity will be a particular challenge for translating this class of drug to the clinic.
Modulation of coactivators and transcriptional regulators of metabolic enzymes, such as PGC-1α, C/EBPs, and others discussed above, could represent an effective method of lowering blood glucose. However, achieving liver specificity when modifying these proteins, potentially through liver-specific PTM regulation or tissue-specific regulation of downstream effectors, would be extremely important given the effects of these proteins on metabolism and other processes in other tissues.
In addition to the potential for hypoglycemia, other possibly detrimental effects of _targeting hepatic glucose production should also be considered, including the risk of redirecting carbons to triglyceride or cholesterol synthesis. This redirection in anaplerotic flux could potentially lead to aberrant increases in lipogenesis and hepatic steatosis, or increased cholesterol. Despite its known effects on inhibiting gluconeogenesis, metformin does not increase triglycerides or blood glucose levels234, suggesting that gluconeogenesis inhibition does not necessarily lead to aberrations in these parameters. However, novel drug _targets would need to be carefully evaluated for their potential effects on triglycerides and cholesterol. Recent technical advances using 13C labeling combined with 13C magnetic resonance spectroscopy could be useful in detecting hepatic fatty acid oxidation in human livers in vivo to determine the effects of candidate drugs on TCA cycle and anaplerotic flux235.
Another potential complication of gluconeogenesis inhibition is lactic acidosis, since anaerobic respiration increases and may lead to lactate accumulation. The incidence of lactic acidosis has been explored with metformin. Since metformin is renally cleared, the drug may accumulate in the kidneys of patients with renal disease, leading to lactic acidosis. However, metformin is not elevated above the therapeutic range in patients with renal failure236. Only a slight increase in lactic acidosis was found to occur with metformin treatment in these patients, with no substantial risk demonstrated in patients with mild to moderate kidney disease. Lactic acidosis is likely not a substantial risk of using drugs to inhibit gluconeogenesis, unless pharmacokinetic data reveals that a particular drug accumulates in tissues above the therapeutic range, which must be tested systematically.
Conclusions and future directions
Despite the existence of several anti-diabetic drugs, type 2 diabetes remains a widespread medical burden. _targeting gluconeogenesis or glucose homeostasis in the liver is an appealing strategy for developing new diabetes therapeutics. Although metformin is currently the most widely used diabetes drug and works at least primarily through inhibition of gluconeogenesis, its mechanism of action has not been fully clarified and it can cause gastrointestinal side effects and typically must be used in combination therapy for long-term sustainability4,11,75. Therefore, there is room for novel therapeutic agents that specifically inhibit hepatic glucose output.
Numerous molecular pathways in the liver contributing to glucose production have the potential to be _targeted pharmacologically in order to decrease blood glucose levels. Efforts to generate drugs that directly inhibit the metabolic enzymes of gluconeogenesis and glycogenolysis are most advanced. There is also a wealth of _targets within additional regulatory pathways, including PGC-1α, FoxOs, CREB, C/EBPs, RERs, RORs, and Srcs, that have the potential to be therapeutically modulated in order to regulate gluconeogenesis. Hepatic glucose output could also be inhibited by administering a liver-specific mitochondrial uncoupler. Although concerns about potency, tissue selectivity, and undesirable metabolic effects such as lactic acidosis must be addressed, further investigation into specific methods for pharmacologically _targeting these pathways in the liver will likely be fruitful in the search for new type 2 diabetes drugs.
Key Points.
Type 2 diabetes is characterized by elevated blood glucose and insulin resistance. Current diabetes drugs can lower blood glucose, but often have side effects, and the most widely used drug metformin does not have a clear mechanism of action.
_targeting glucose and glycogen metabolism in the liver is a strategy for type 2 diabetes treatment which can decrease hepatic glucose output, but this approach has not been fully explored.
_targeting gluconeogenic and glycogenolytic enzymes or their regulators presents numerous drug _targets that are currently in development or have the potential to be developed.
Transcriptional coactivators and transcription factors are emerging diabetes drug _targets with the ability to control entire gene programs involved in glucose and glycogen metabolism. These transcriptional regulators may be able to be _targeted more specifically by modulating their protein-protein interactions or post-translational modifications.
Novel diabetes drugs would most likely be used in combination with existing therapies to enable sustained blood glucose suppression, and so that each drug could be used at a lower concentration in order to reduce side effects. Drugs decreasing hepatic glucose output may be most effectively used with drugs that work by other mechanisms, such as thiazolidinediones or SGLT2 inhibitors.
Challenges of inhibiting hepatic glucose output include preventing hypoglycemia, enabling tissue-specific _targeting, analyzing the possible effects of redirecting carbons to triglyceride or cholesterol synthesis, and avoiding lactic acidosis.
Acknowledgments
Amy K. Rines received funding from the NIH NIDDK (F32 DK102293-01). Kfir Sharabi was funded by the American Heart Association (15POST22880002). Clint D. J. Tavares received funding from the American Diabetes Association (1-16-PDF-111). Pere Puigserver was funded by the NIH NIDDK (R01 DK069966, DK081418, DK089883) and the American Diabetes Association.
Glossary Terms
- Type 2 diabetes
Chronic disease of aberrant glucose homeostasis that is characterized by elevated blood glucose and insulin resistance
- Hyperglycemia
Elevated blood glucose above normal levels
- Hypoglycemia
Suppressed blood glucose below normal levels
- Gluconeogenesis
Metabolic pathway utilizing carbon substrates to generate glucose
- Glycogenolysis
Metabolic pathway that breaks down glycogen into glucose
- Hepatic steatosis
Fatty liver disease
- Hyperinsulinemia
Excess insulin levels in the blood, often caused by insulin resistance
- Lactic acidosis
Increased acidity in the body due to a buildup of lactate, a potential concern with inhibiting hepatic gluconeogenesis
- Sulfonylureas
Type 2 diabetes drugs that stimulate insulin secretion from the pancreas through inhibition of KATP channels in beta cells
- Thiazolidinediones
Insulin-sensitizing type 2 diabetes drugs that act as PPARY agonists, also known as glitazones
- Insulin secretagogue
Any agent that increases insulin release from the pancreas
Biographies
Amy K. Rines, Ph.D., earned her B.A. in Integrated Science and Biology and Ph.D. in Cell Biology from Northwestern University, where her doctoral research focused on kinase regulation of cell proliferation and metabolism. She joined Pere Puigserver’s group at Dana-Farber Cancer Institute and Harvard Medical School as a postdoctoral fellow to pursue her research interests of discovering cellular signaling mechanisms and therapeutics for type 2 diabetes and obesity.
Kfir Sharabi, Ph.D., completed his graduate studies at The Hebrew University of Jerusalem in genetics studying the molecular response of worms to environmental changes in CO2 levels. He is currently pursuing postdoctoral training where he uses a chemical approach to study how glucose homeostasis is regulated in diabetic states. His main research interests include studying how nutrients affect specific metabolic pathways to control energy homeostasis.
Clint D. J. Tavares, Ph.D., is currently a postdoctoral research fellow in the laboratory of Pere Puigserver at the Dana-Farber Cancer Institute and Harvard Medical School. Supported by a postdoctoral fellowship from The American Diabetes Association, his research is aimed at investigating certain molecular components, mechanisms, and transduction pathways involved in nutrient-regulated metabolic responses in the context of diabetes, with the objective of chemically _targeting specific components for the therapeutic purpose of treating type 2 diabetes and obesity. He previously received his Ph.D. in Cell and Molecular Biology from The University of Texas at Austin, under the guidance of Kevin N. Dalby.
Pere Puigserver, Ph.D., received his B.S. in Biological Sciences and Ph.D. in Biochemistry at the University of Illes Balears, Spain. His graduate work focused on mitochondrial energetics using in vitro and in vivo approaches, and included research at the University of Stockholm. He pursued postdoctoral training in molecular and cellular biology at Dana-Farber Cancer Institute and Harvard Medical School. In 2002, he was appointed Assistant Professor of Cell Biology at Johns Hopkins University School of Medicine. In 2006, he was recruited back to the Dana-Farber Cancer Institute and Harvard Medical School to continue his research program in cell metabolism.
Footnotes
Competing Interests Statement
The authors declare no competing financial interests.
References
- 1.Centers for Disease Control and Prevention. Atlanta, ga: US Department of health and human services; 2014. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. [Google Scholar]
- 2.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. The New England journal of medicine. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 4.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell metabolism. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. This work reviews the complexities surrounding the mechanism of action of metformin, the most widely used anti-diabetic drug. [DOI] [PubMed] [Google Scholar]
- 5.Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 7.Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell metabolism. 2014;20:573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derosa G, Maffioli P. Efficacy and safety profile evaluation of acarbose alone and in association with other antidiabetic drugs: a systematic review. Clinical therapeutics. 2012;34:1221–1236. doi: 10.1016/j.clinthera.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Chao EC, Henry RR. SGLT2 inhibition--a novel strategy for diabetes treatment. Nature reviews. Drug discovery. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 10.Handelsman Y. Role of bile acid sequestrants in the treatment of type 2 diabetes. Diabetes care. 2011;34(Suppl 2):S244–250. doi: 10.2337/dc11-s237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchoucha M, Uzzan B, Cohen R. Metformin and digestive disorders. Diabetes & metabolism. 2011;37:90–96. doi: 10.1016/j.diabet.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Del Prato S, Pulizzi N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism: clinical and experimental. 2006;55:S20–27. doi: 10.1016/j.metabol.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Despres JP, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. The New England journal of medicine. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher EJ, LeRoith D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes care. 2013;36(Suppl 2):S233–239. doi: 10.2337/dcS13-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postic C, Dentin R, Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes & metabolism. 2004;30:398–408. doi: 10.1016/s1262-3636(07)70133-7. [DOI] [PubMed] [Google Scholar]
- 16.Meyer C, Dostou JM, Welle SL, Gerich JE. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. American journal of physiology. Endocrinology and metabolism. 2002;282:E419–427. doi: 10.1152/ajpendo.00032.2001. [DOI] [PubMed] [Google Scholar]
- 17.Kiyosue A, Hayashi N, Komori H, Leonsson-Zachrisson M, Johnsson E. Dose-ranging study with the glucokinase activator AZD1656 as monotherapy in Japanese patients with type 2 diabetes mellitus. Diabetes, obesity & metabolism. 2013;15:923–930. doi: 10.1111/dom.12100. [DOI] [PubMed] [Google Scholar]
- 18.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nature reviews. Drug discovery. 2009;8:399–416. doi: 10.1038/nrd2850. This review discusses the development of glucokinase activators as drugs for type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- 19.Erion DM, et al. The hepatoselective glucokinase activator PF-04991532 ameliorates hyperglycemia without causing hepatic steatosis in diabetic rats. PloS one. 2014;9:e97139. doi: 10.1371/journal.pone.0097139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma R, et al. Comparison of the circulating metabolite profile of PF-04991532, a hepatoselective glucokinase activator, across preclinical species and humans: potential implications in metabolites in safety testing assessment. Drug metabolism and disposition: the biological fate of chemicals. 2015;43:190–198. doi: 10.1124/dmd.114.061218. [DOI] [PubMed] [Google Scholar]
- 21.Katz L, et al. AMG 151 (ARRY-403), a novel glucokinase activator, decreases fasting and postprandial glycaemia in patients with type 2 diabetes. Diabetes, obesity & metabolism. 2016;18:191–195. doi: 10.1111/dom.12586. [DOI] [PubMed] [Google Scholar]
- 22.Zhi J, Zhai S. Effects of piragliatin, a glucokinase activator, on fasting and postprandial plasma glucose in patients with type 2 diabetes mellitus. Journal of clinical pharmacology. 2016;56:231–238. doi: 10.1002/jcph.589. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd DJ, et al. Antidiabetic effects of glucokinase regulatory protein small-molecule disruptors. Nature. 2013;504:437–440. doi: 10.1038/nature12724. The authors identify two small molecule inhibitors of the GK-GKRP interaction, and their ability to lower blood glucose in diabetic rodents. [DOI] [PubMed] [Google Scholar]
- 24.Valcarce C. The Importance of Tissue Selectivity and Preservation of the Physiological Regulation when _targeting Key Metabolic Regulators as Glucokinase; Keystone Symposia on New Therapeutics for Diabetes and Obesity; La Jolla, CA. April 18-20, 2016. [Google Scholar]
- 25.Agius L. New hepatic _targets for glycaemic control in diabetes. Best practice & research. Clinical endocrinology & metabolism. 2007;21:587–605. doi: 10.1016/j.beem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Oikonomakos NG. Glycogen phosphorylase as a molecular _target for type 2 diabetes therapy. Current protein & peptide science. 2002;3:561–586. doi: 10.2174/1389203023380422. [DOI] [PubMed] [Google Scholar]
- 27.Treadway JL, Mendys P, Hoover DJ. Glycogen phosphorylase inhibitors for treatment of type 2 diabetes mellitus. Expert opinion on investigational drugs. 2001;10:439–454. doi: 10.1517/13543784.10.3.439. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa AK, et al. Glucose-lowering in a db/db mouse model by dihydropyridine diacid glycogen phosphorylase inhibitors. Bioorganic & medicinal chemistry letters. 2003;13:3405–3408. doi: 10.1016/s0960-894x(03)00798-4. [DOI] [PubMed] [Google Scholar]
- 29.Martin WH, et al. Discovery of a human liver glycogen phosphorylase inhibitor that lowers blood glucose in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1776–1781. doi: 10.1073/pnas.95.4.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rath VL, et al. Human liver glycogen phosphorylase inhibitors bind at a new allosteric site. Chemistry & biology. 2000;7:677–682. doi: 10.1016/s1074-5521(00)00004-1. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa S, Murakami K, Nishikawa M, Nakayama O, Hino M. FR258900, a novel glycogen phosphorylase inhibitor isolated from Fungus No. 138354. II. Anti-hyperglycemic effects in diabetic animal models. The Journal of antibiotics. 2005;58:503–506. doi: 10.1038/ja.2005.67. [DOI] [PubMed] [Google Scholar]
- 32.Suh SW, et al. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmethyl)pro pyl]-1H–indole-2-carboxamide) The Journal of pharmacology and experimental therapeutics. 2007;321:45–50. doi: 10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- 33.Torres TP, et al. Impact of a glycogen phosphorylase inhibitor and metformin on basal and glucagon-stimulated hepatic glucose flux in conscious dogs. The Journal of pharmacology and experimental therapeutics. 2011;337:610–620. doi: 10.1124/jpet.110.177899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flattem N, et al. Alpha- and beta-cell responses to small changes in plasma glucose in the conscious dog. Diabetes. 2001;50:367–375. doi: 10.2337/diabetes.50.2.367. [DOI] [PubMed] [Google Scholar]
- 35.Kazierad DJ, et al. Effects of multiple ascending doses of the glucagon receptor antagonist, PF-06291874, in patients with type 2 diabetes mellitus. Diabetes, obesity & metabolism. 2016 doi: 10.1111/dom.12672. [DOI] [PubMed] [Google Scholar]
- 36.Vajda EJPDJ, Logan DK, Li Y, Orloff DJ, Pipkin JD, Zhi L, Marschke KB. Pharmacodynamic Effects of Single Doses of the Glucagon Receptor Antagonist LGD-6972 in Healthy Subjects and Subjects with Type 2 Diabetes Mellitus (T2DM); Endocrine Society’s 97th Annual Meeting and Expo; San Diego, CA. March 5-8, 2015. [Google Scholar]
- 37.Petersen KF, Sullivan JT. Effects of a novel glucagon receptor antagonist (Bay 27–9955) on glucagon-stimulated glucose production in humans. Diabetologia. 2001;44:2018–2024. doi: 10.1007/s001250100006. [DOI] [PubMed] [Google Scholar]
- 38.Guan HP, et al. Glucagon receptor antagonism induces increased cholesterol absorption. Journal of lipid research. 2015;56:2183–2195. doi: 10.1194/jlr.M060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong Y, et al. Discovery of a novel glucagon receptor antagonist N-[(4-{(1S)-1-[3-(3, 5-dichlorophenyl)-5-(6-methoxynaphthalen-2-yl)-1H–pyrazol-1-yl]ethyl}phenyl)carbo nyl]-beta-alanine (MK-0893) for the treatment of type II diabetes. Journal of medicinal chemistry. 2012;55:6137–6148. doi: 10.1021/jm300579z. [DOI] [PubMed] [Google Scholar]
- 40.Kelly RP, et al. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes, obesity & metabolism. 2015;17:414–422. doi: 10.1111/dom.12446. [DOI] [PubMed] [Google Scholar]
- 41.Kazda CM, et al. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of the Glucagon Receptor Antagonist LY2409021 in Patients With Type 2 Diabetes. Diabetes care. 2015 [Google Scholar]
- 42.van Poelje PD, et al. Inhibition of fructose 1,6-bisphosphatase reduces excessive endogenous glucose production and attenuates hyperglycemia in Zucker diabetic fatty rats. Diabetes. 2006;55:a1747–1754. doi: 10.2337/db05-1443. [DOI] [PubMed] [Google Scholar]
- 43.Dang Q, et al. Fructose-1, 6-bisphosphatase Inhibitors. 2, Design, synthesis, and structure-activity relationship of a series of phosphonic acid containing benzimidazoles that function as 5’-adenosinemonophosphate (AMP) mimics. Journal of medicinal chemistry. 2010;53:441–451. doi: 10.1021/jm901420x. [DOI] [PubMed] [Google Scholar]
- 44.Aicher TD, Boyd SA, McVean M, Celeste A. Novel therapeutics and _targets for the treatment of diabetes. Expert review of clinical pharmacology. 2010;3:209–229. doi: 10.1586/ecp.10.1. [DOI] [PubMed] [Google Scholar]
- 45.Liu G. Technology evaluation: ISIS-113715, Isis. Current opinion in molecular therapeutics. 2004;6:331–336. [PubMed] [Google Scholar]
- 46.Rondinone CM, et al. Protein tyrosine phosphatase 1B reduction regulates adiposity and expression of genes involved in lipogenesis. Diabetes. 2002;51:2405–2411. doi: 10.2337/diabetes.51.8.2405. [DOI] [PubMed] [Google Scholar]
- 47.Swarbrick MM, et al. Inhibition of protein tyrosine phosphatase-1B with antisense oligonucleotides improves insulin sensitivity and increases adiponectin concentrations in monkeys. Endocrinology. 2009;150:1670–1679. doi: 10.1210/en.2008-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wrobel J, et al. PTP1B inhibition and antihyperglycemic activity in the ob/ob mouse model of novel 11-arylbenzo[b]naphtho[2,3-d]furans and 11-arylbenzo[b]naphtho[2,3-d]thiophenes. Journal of medicinal chemistry. 1999;42:3199–3202. doi: 10.1021/jm990260v. [DOI] [PubMed] [Google Scholar]
- 49.Erbe DV, et al. Ertiprotafib improves glycemic control and lowers lipids via multiple mechanisms. Molecular pharmacology. 2005;67:69–77. doi: 10.1124/mol.104.005553. [DOI] [PubMed] [Google Scholar]
- 50.Shrestha S, Bhattarai BR, Cho H, Choi JK, Cho H. PTP1B inhibitor Ertiprotafib is also a potent inhibitor of IkappaB kinase beta (IKK-beta) Bioorganic & medicinal chemistry letters. 2007;17:2728–2730. doi: 10.1016/j.bmcl.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Zasloff M, et al. A spermine-coupled cholesterol metabolite from the shark with potent appetite suppressant and antidiabetic properties. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25:689–697. doi: 10.1038/sj.ijo.0801599. [DOI] [PubMed] [Google Scholar]
- 52.Lantz KA, et al. Inhibition of PTP1B by trodusquemine (MSI-1436) causes fat-specific weight loss in diet-induced obese mice. Obesity. 2010;18:1516–1523. doi: 10.1038/oby.2009.444. [DOI] [PubMed] [Google Scholar]
- 53.Cho H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitamins and hormones. 2013;91:405–424. doi: 10.1016/B978-0-12-407766-9.00017-1. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan N, et al. _targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nature chemical biology. 2014;10:558–566. doi: 10.1038/nchembio.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bano G. Glucose homeostasis, obesity and diabetes. Best practice & research. Clinical obstetrics & gynaecology. 2013;27:715–726. doi: 10.1016/j.bpobgyn.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Cano N. Bench-to-bedside review: glucose production from the kidney. Critical care. 2002;6:317–321. doi: 10.1186/cc1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mithieux G, Andreelli F, Magnan C. Intestinal gluconeogenesis: key signal of central control of energy and glucose homeostasis. Current opinion in clinical nutrition and metabolic care. 2009;12:419–423. doi: 10.1097/MCO.0b013e32832c4d6a. [DOI] [PubMed] [Google Scholar]
- 58.Radziuk J, Pye S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes/metabolism research and reviews. 2001;17:250–272. doi: 10.1002/dmrr.217. [DOI] [PubMed] [Google Scholar]
- 59.Taylor SI. Deconstructing type 2 diabetes. Cell. 1999;97:9–12. doi: 10.1016/s0092-8674(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 60.Newgard CB, Hirsch LJ, Foster DW, McGarry JD. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? The Journal of biological chemistry. 1983;258:8046–8052. [PubMed] [Google Scholar]
- 61.Moore MC, et al. Sources of carbon for hepatic glycogen synthesis in the conscious dog. The Journal of clinical investigation. 1991;88:578–587. doi: 10.1172/JCI115342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edgerton DS, et al. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. The Journal of clinical investigation. 2006;116:521–527. doi: 10.1172/JCI27073. This study utilizes a normal dog model to demonstrate that the direct effects of insulin predominately regulate glucose production in the liver, while insulin action at the hypothalamus has negligible effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thiebaud D, et al. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982;31:957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- 64.Dobbins RL, et al. Role of glucagon in countering hypoglycemia induced by insulin infusion in dogs. The American journal of physiology. 1991;261:E773–781. doi: 10.1152/ajpendo.1991.261.6.E773. [DOI] [PubMed] [Google Scholar]
- 65.Landau BR, et al. Contributions of gluconeogenesis to glucose production in the fasted state. The Journal of clinical investigation. 1996;98:378–385. doi: 10.1172/JCI118803. This work studied healthy human subjects to conclude that gluconeogenesis contributes to 50% of glucose production after an overnight fast, and nearly all of glucose production within the following two days. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991;254:573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- 67.Weber G, Singhal RL, Stamm NB, Fisher EA, Mentendiek MA. Regulation of enzymes involved in gluconeogenesis. Advances in enzyme regulation. 1964;2:1–38. doi: 10.1016/s0065-2571(64)80003-0. [DOI] [PubMed] [Google Scholar]
- 68.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes care. 2006;29:1130–1139. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 69.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D’Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes, obesity & metabolism. 2011;13(Suppl 1):126–132. doi: 10.1111/j.1463-1326.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- 71.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. The Journal of clinical investigation. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wajngot A, et al. Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus. Metabolism: clinical and experimental. 2001;50:47–52. doi: 10.1053/meta.2001.19422. [DOI] [PubMed] [Google Scholar]
- 73.Hwang JH, et al. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. The Journal of clinical investigation. 1995;95:783–787. doi: 10.1172/JCI117727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nature reviews. Drug discovery. 2009;8:417–429. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. Jama. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 76.Pfefferkorn JA, et al. Discovery of (S)-6-(3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanamido)nicotini c acid as a hepatoselective glucokinase activator clinical candidate for treating type 2 diabetes mellitus. Journal of medicinal chemistry. 2012;55:1318–1333. doi: 10.1021/jm2014887. [DOI] [PubMed] [Google Scholar]
- 77.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nature reviews. Molecular cell biology. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 78.Cusi K, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. The Journal of clinical investigation. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elchebly M, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. The authors use knockout mice to demonstrate that PTP-1b lowers blood glucose, enhances insulin sensitivity, and provides resistance to weight gain, identifying PTP-1b as a potential _target for type 2 diabetes treatment. [DOI] [PubMed] [Google Scholar]
- 80.Delibegovic M, et al. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes. 2009;58:590–599. doi: 10.2337/db08-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Owen C, et al. Inducible liver-specific knockdown of protein tyrosine phosphatase 1B improves glucose and lipid homeostasis in adult mice. Diabetologia. 2013;56:2286–2296. doi: 10.1007/s00125-013-2992-z. [DOI] [PubMed] [Google Scholar]
- 82.Chen PJ, Cai SP, Huang C, Meng XM, Li J. Protein tyrosine phosphatase 1B (PTP1B): A key regulator and therapeutic _target in liver diseases. Toxicology. 2015;337:10–20. doi: 10.1016/j.tox.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 83.Krssak M, et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes. 2004;53:3048–3056. doi: 10.2337/diabetes.53.12.3048. [DOI] [PubMed] [Google Scholar]
- 84.Cline GW, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. The New England journal of medicine. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- 85.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. The Journal of clinical investigation. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Furukawa S, et al. FR258900, a novel glycogen phosphorylase inhibitor isolated from Fungus No. 138354. I. Taxonomy, fermentation, isolation and biological activities. The Journal of antibiotics. 2005;58:497–502. doi: 10.1038/ja.2005.66. [DOI] [PubMed] [Google Scholar]
- 87.Tiraidis C, et al. FR258900, a potential anti-hyperglycemic drug, binds at the allosteric site of glycogen phosphorylase. Protein science : a publication of the Protein Society. 2007;16:1773–1782. doi: 10.1110/ps.072925607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Somsak L, et al. New inhibitors of glycogen phosphorylase as potential antidiabetic agents. Current medicinal chemistry. 2008;15:2933–2983. doi: 10.2174/092986708786848659. [DOI] [PubMed] [Google Scholar]
- 89.Baker DJ, Timmons JA, Greenhaff PL. Glycogen phosphorylase inhibition in type 2 diabetes therapy: a systematic evaluation of metabolic and functional effects in rat skeletal muscle. Diabetes. 2005;54:2453–2459. doi: 10.2337/diabetes.54.8.2453. [DOI] [PubMed] [Google Scholar]
- 90.Lu Z, et al. A new class of glycogen phosphorylase inhibitors. Bioorganic & medicinal chemistry letters. 2003;13:4125–4128. doi: 10.1016/j.bmcl.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 91.Tracey WR, et al. Cardioprotective effects of ingliforib, a novel glycogen phosphorylase inhibitor. American journal of physiology. Heart and circulatory physiology. 2004;286:H1177–1184. doi: 10.1152/ajpheart.00652.2003. [DOI] [PubMed] [Google Scholar]
- 92.Hers HG. The control of glycogen metabolism in the liver. Annual review of biochemistry. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- 93.Hutson NJ, Brumley FT, Assimacopoulos FD. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. The Journal of biological chemistry. 1976;251:5200–5208. [PubMed] [Google Scholar]
- 94.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. European journal of biochemistry / FEBS. 1980;107:519–527. [PubMed] [Google Scholar]
- 95.Nikoulina SE, et al. Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes. 2000;49:263–271. doi: 10.2337/diabetes.49.2.263. [DOI] [PubMed] [Google Scholar]
- 96.Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG. Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes. 1999;48:1662–1666. doi: 10.2337/diabetes.48.8.1662. [DOI] [PubMed] [Google Scholar]
- 97.Coghlan MP, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chemistry & biology. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 98.Lochhead PA, Coghlan M, Rice SQ, Sutherland C. Inhibition of GSK-3 selectively reduces glucose-6-phosphatase and phosphatase and phosphoenolypyruvate carboxykinase gene expression. Diabetes. 2001;50:937–946. doi: 10.2337/diabetes.50.5.937. [DOI] [PubMed] [Google Scholar]
- 99.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nature reviews. Drug discovery. 2004;3:479–487. doi: 10.1038/nrd1415. This review discusses the biology of GSK3 inhibitors and their development as treatment for type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- 100.Cline GW, et al. Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (fa/fa) rats. Diabetes. 2002;51:2903–2910. doi: 10.2337/diabetes.51.10.2903. [DOI] [PubMed] [Google Scholar]
- 101.Ring DB, et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52:588–595. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- 102.Kaidanovich-Beilin O, Eldar-Finkelman H. Long-term treatment with novel glycogen synthase kinase-3 inhibitor improves glucose homeostasis in ob/ob mice: molecular characterization in liver and muscle. The Journal of pharmacology and experimental therapeutics. 2006;316:17–24. doi: 10.1124/jpet.105.090266. [DOI] [PubMed] [Google Scholar]
- 103.Eldar-Finkelman H, Martinez A. GSK-3 Inhibitors: Preclinical and Clinical Focus on CNS. Frontiers in molecular neuroscience. 2011;4:32. doi: 10.3389/fnmol.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gomez-Valades AG, et al. Overcoming diabetes-induced hyperglycemia through inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP) with RNAi. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;13:401–410. doi: 10.1016/j.ymthe.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 105.Georgievska B, et al. AZD1080, a novel GSK3 inhibitor, rescues synaptic plasticity deficits in rodent brain and exhibits peripheral _target engagement in humans. Journal of neurochemistry. 2013;125:446–456. doi: 10.1111/jnc.12203. [DOI] [PubMed] [Google Scholar]
- 106.Tolosa E, et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Movement disorders : official journal of the Movement Disorder Society. 2014;29:470–478. doi: 10.1002/mds.25824. [DOI] [PubMed] [Google Scholar]
- 107.Dominguez JM, et al. Evidence for irreversible inhibition of glycogen synthase kinase-3beta by tideglusib. The Journal of biological chemistry. 2012;287:893–904. doi: 10.1074/jbc.M111.306472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36:274–283. doi: 10.2337/diab.36.3.274. [DOI] [PubMed] [Google Scholar]
- 109.Matsuda M, et al. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism: clinical and experimental. 2002;51:1111–1119. doi: 10.1053/meta.2002.34700. [DOI] [PubMed] [Google Scholar]
- 110.Sorensen H, et al. Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes. 2006;55:2843–2848. doi: 10.2337/db06-0222. [DOI] [PubMed] [Google Scholar]
- 111.O’Harte FP, Franklin ZJ, Irwin N. Two novel glucagon receptor antagonists prove effective therapeutic agents in high-fat-fed and obese diabetic mice. Diabetes, obesity & metabolism. 2014;16:1214–1222. doi: 10.1111/dom.12360. [DOI] [PubMed] [Google Scholar]
- 112.Qureshi SA, et al. A novel glucagon receptor antagonist inhibits glucagon-mediated biological effects. Diabetes. 2004;53:3267–3273. doi: 10.2337/diabetes.53.12.3267. [DOI] [PubMed] [Google Scholar]
- 113.Rivera N, et al. A novel glucagon receptor antagonist, NNC 25–0926, blunts hepatic glucose production in the conscious dog. The Journal of pharmacology and experimental therapeutics. 2007;321:743–752. doi: 10.1124/jpet.106.115717. [DOI] [PubMed] [Google Scholar]
- 114.Perry RJ, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. doi: 10.1016/j.cell.2015.01.012. The authors discover that insulin can decrease hepatic gluconeogenesis and protect against high fat diet-related hyperglycemia through inhibition of lipolysis in white adipose tissue, which decreases acetyl CoA and pyruvate carboxylase activity in the liver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kumashiro N, et al. _targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes. 2013;62:2183–2194. doi: 10.2337/db12-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bahl JJ, Matsuda M, DeFronzo RA, Bressler R. In vitro and in vivo suppression of gluconeogenesis by inhibition of pyruvate carboxylase. Biochemical pharmacology. 1997;53:67–74. doi: 10.1016/s0006-2952(96)00660-0. [DOI] [PubMed] [Google Scholar]
- 117.Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- 118.McCommis KS, Finck BN. Mitochondrial pyruvate transport: a historical perspective and future research directions. The Biochemical journal. 2015;466:443–454. doi: 10.1042/BJ20141171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bricker DK, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Herzig S, et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 121.Gray LR, et al. Hepatic Mitochondrial Pyruvate Carrier 1 Is Required for Efficient Regulation of Gluconeogenesis and Whole-Body Glucose Homeostasis. Cell metabolism. 2015;22:669–681. doi: 10.1016/j.cmet.2015.07.027. This work demonstrates that the mitochondrial pyruvate carrier contributes significantly to pyruvate-driven gluconeogenesis and hyperglycemia in type 2 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCommis KS, et al. Loss of Mitochondrial Pyruvate Carrier 2 in the Liver Leads to Defects in Gluconeogenesis and Compensation via Pyruvate-Alanine Cycling. Cell metabolism. 2015;22:682–694. doi: 10.1016/j.cmet.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Divakaruni AS, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Colca JR, et al. Identification of a mitochondrial _target of thiazolidinedione insulin sensitizers (mTOT)--relationship to newly identified mitochondrial pyruvate carrier proteins. PloS one. 2013;8:e61551. doi: 10.1371/journal.pone.0061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vacanti NM, et al. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Molecular cell. 2014;56:425–435. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. American journal of physiology. Endocrinology and metabolism. 2003;285:E685–692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- 127.Cimbala MA, et al. Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney. Effects of insulin and cyclic AMP. The Journal of biological chemistry. 1982;257:7629–7636. [PubMed] [Google Scholar]
- 128.Granner D, Andreone T, Sasaki K, Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983;305:549–551. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- 129.Magnuson MA, Quinn PG, Granner DK. Multihormonal regulation of phosphoenolpyruvate carboxykinase-chloramphenicol acetyltransferase fusion genes. Insulin’s effects oppose those of cAMP and dexamethasone. The Journal of biological chemistry. 1987;262:14917–14920. [PubMed] [Google Scholar]
- 130.Valera A, Pujol A, Pelegrin M, Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9151–9154. doi: 10.1073/pnas.91.19.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DiTullio NW, et al. 3-mercaptopicolinic acid, an inhibitor of gluconeogenesis. The Biochemical journal. 1974;138:387–394. doi: 10.1042/bj1380387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Samuel VT, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12121–12126. doi: 10.1073/pnas.0812547106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hakimi P, et al. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutrition & metabolism. 2005;2:33. doi: 10.1186/1743-7075-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.She P, et al. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Molecular and cellular biology. 2000;20:6508–6517. doi: 10.1128/mcb.20.17.6508-6517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burgess SC, et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. The Journal of biological chemistry. 2004;279:48941–48949. doi: 10.1074/jbc.M407120200. [DOI] [PubMed] [Google Scholar]
- 136.Burgess SC, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell metabolism. 2007;5:313–320. doi: 10.1016/j.cmet.2007.03.004. The authors find that a 90% reduction in hepatic PEPCK in mice results in only a 40% decrease in gluconeogenic flux, demonstrating that the control of PEPCK alone on gluconeogenesis is not as strong as expected, and that PEPCK may need to work together with hepatic energy production to control gluconeogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mutel E, et al. Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: induction of renal and intestinal gluconeogenesis by glucagon. Diabetes. 2011;60:3121–3131. doi: 10.2337/db11-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abdul-Wahed A, et al. A link between hepatic glucose production and peripheral energy metabolism via hepatokines. Molecular metabolism. 2014;3:531–543. doi: 10.1016/j.molmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rhee J, et al. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Codogno P, Meijer AJ. Autophagy in the liver. Journal of hepatology. 2013;59:389–391. doi: 10.1016/j.jhep.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 141.Okar DA, Lange AJ. Fructose-2,6-bisphosphate and control of carbohydrate metabolism in eukaryotes. BioFactors. 1999;10:1–14. doi: 10.1002/biof.5520100101. [DOI] [PubMed] [Google Scholar]
- 142.Wu C, Okar DA, Newgard CB, Lange AJ. Increasing fructose 2,6-bisphosphate overcomes hepatic insulin resistance of type 2 diabetes. American journal of physiology. Endocrinology and metabolism. 2002;282:E38–45. doi: 10.1152/ajpendo.2002.282.1.E38. [DOI] [PubMed] [Google Scholar]
- 143.Wu C, Okar DA, Newgard CB, Lange AJ. Overexpression of 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase in mouse liver lowers blood glucose by suppressing hepatic glucose production. The Journal of clinical investigation. 2001;107:91–98. doi: 10.1172/JCI11103. This study overexpressed 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase in normal and diabetic mice, which resulted in decreased blood glucose and hepatic glucose production and suggested that this enzyme may be a _target for type 2 diabetes treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cochran AG. Antagonists of protein-protein interactions. Chemistry & biology. 2000;7:R85–94. doi: 10.1016/s1074-5521(00)00106-x. [DOI] [PubMed] [Google Scholar]
- 145.Song X, et al. Development of potent small-molecule inhibitors to drug the undruggable steroid receptor coactivator-3. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:4970–4975. doi: 10.1073/pnas.1604274113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fontaine F, Overman J, Francois M. Pharmacological manipulation of transcription factor protein-protein interactions: opportunities and obstacles. Cell regeneration. 2015;4:2. doi: 10.1186/s13619-015-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thompson AD, Dugan A, Gestwicki JE, Mapp AK. Fine-tuning multiprotein complexes using small molecules. ACS chemical biology. 2012;7:1311–1320. doi: 10.1021/cb300255p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bhagwat AS, Vakoc CR. _targeting Transcription Factors in Cancer. Trends in cancer. 2015;1:53–65. doi: 10.1016/j.trecan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Darnell JE., Jr Transcription factors as _targets for cancer therapy. Nature reviews. Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 150.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Advances in physiology education. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 151.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 152.Esterbauer H, Oberkofler H, Krempler F, Patsch W. Human peroxisome proliferator activated receptor gamma coactivator 1 (PPARGC1) gene: cDNA sequence, genomic organization, chromosomal localization, and tissue expression. Genomics. 1999;62:98–102. doi: 10.1006/geno.1999.5977. [DOI] [PubMed] [Google Scholar]
- 153.Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 154.Yoon JC, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. The authors identify that PGC-1α is strongly induced by fasting in mouse livers, and activates the gluconeogenic program and glucose output in hepatocytes. [DOI] [PubMed] [Google Scholar]
- 155.Puigserver P, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. This work demonstrates that FOXO1 is required for PGC-1α to robustly induce the insulin-regulated gluconeogenic gene program. [DOI] [PubMed] [Google Scholar]
- 156.Koo SH, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nature medicine. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 157.Leone TC, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS biology. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lin J, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 159.Estall JL, et al. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression. Diabetes. 2009;58:1499–1508. doi: 10.2337/db08-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Burgess SC, et al. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. The Journal of biological chemistry. 2006;281:19000–19008. doi: 10.1074/jbc.M600050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 162.Rodgers JT, Haas W, Gygi SP, Puigserver P. Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell metabolism. 2010;11:23–34. doi: 10.1016/j.cmet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dominy JE, Jr, Lee Y, Gerhart-Hines Z, Puigserver P. Nutrient-dependent regulation of PGC-1alpha’s acetylation state and metabolic function through the enzymatic activities of Sirt1/GCN5. Biochimica et biophysica acta. 2010;1804:1676–1683. doi: 10.1016/j.bbapap.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 165.Lerin C, et al. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell metabolism. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 166.Dominy JE, Jr, et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Molecular cell. 2012;48:900–913. doi: 10.1016/j.molcel.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee Y, et al. Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature. 2014;510:547–551. doi: 10.1038/nature13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Teyssier C, Ma H, Emter R, Kralli A, Stallcup MR. Activation of nuclear receptor coactivator PGC-1alpha by arginine methylation. Genes & development. 2005;19:1466–1473. doi: 10.1101/gad.1295005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lustig Y, et al. Separation of the gluconeogenic and mitochondrial functions of PGC-1{alpha} through S6 kinase. Genes & development. 2011;25:1232–1244. doi: 10.1101/gad.2054711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sakai M, et al. CITED2 links hormonal signaling to PGC-1alpha acetylation in the regulation of gluconeogenesis. Nature medicine. 2012;18:612–617. doi: 10.1038/nm.2691. [DOI] [PubMed] [Google Scholar]
- 171.Dekker FJ, van den Bosch T, Martin NI. Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases. Drug discovery today. 2014;19:654–660. doi: 10.1016/j.drudis.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 172.Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nature reviews. Cancer. 2009;9:123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature cell biology. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends in endocrinology and metabolism: TEM. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. The Journal of clinical investigation. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Arany Z, et al. Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1alpha and oxidative phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4721–4726. doi: 10.1073/pnas.0800979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang LN, et al. Novel small-molecule PGC-1alpha transcriptional regulator with beneficial effects on diabetic db/db mice. Diabetes. 2013;62:1297–1307. doi: 10.2337/db12-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Burgering BM. A brief introduction to FOXOlogy. Oncogene. 2008;27:2258–2262. doi: 10.1038/onc.2008.29. [DOI] [PubMed] [Google Scholar]
- 179.Haeusler RA, Kaestner KH, Accili D. FoxOs function synergistically to promote glucose production. The Journal of biological chemistry. 2010;285:35245–35248. doi: 10.1074/jbc.C110.175851. This work demonstrates that ablation of hepatic FOXO2 and FOXO3 with FOXO1 lowers blood glucose and increases insulin sensitivity compared to loss of FOXO1 alone, suggesting that FOXO isoforms work together to promote hepatic gluconeogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim DH, et al. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes. 2011;60:2763–2774. doi: 10.2337/db11-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tikhanovich I, Cox J, Weinman SA. Forkhead box class O transcription factors in liver function and disease. Journal of gastroenterology and hepatology. 2013;28(Suppl 1):125–131. doi: 10.1111/jgh.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang K, et al. Hepatic suppression of Foxo1 and Foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology. 2012;153:631–646. doi: 10.1210/en.2011-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tao R, et al. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. The Journal of biological chemistry. 2011;286:14681–14690. doi: 10.1074/jbc.M110.201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 185.Sajan MP, et al. Akt-dependent phosphorylation of hepatic FoxO1 is compartmentalized on a WD40/ProF scaffold and is selectively inhibited by aPKC in early phases of diet-induced obesity. Diabetes. 2014;63:2690–2701. doi: 10.2337/db13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hall JA, Tabata M, Rodgers JT, Puigserver P. USP7 attenuates hepatic gluconeogenesis through modulation of FoxO1 gene promoter occupancy. Molecular endocrinology. 2014;28:912–924. doi: 10.1210/me.2013-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kato S, Ding J, Pisck E, Jhala US, Du K. COP1 functions as a FoxO1 ubiquitin E3 ligase to regulate FoxO1-mediated gene expression. The Journal of biological chemistry. 2008;283:35464–35473. doi: 10.1074/jbc.M801011200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mihaylova MM, et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nagashima T, et al. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Molecular pharmacology. 2010;78:961–970. doi: 10.1124/mol.110.065714. [DOI] [PubMed] [Google Scholar]
- 190.Tanaka H, et al. Effects of the novel Foxo1 inhibitor AS1708727 on plasma glucose and triglyceride levels in diabetic db/db mice. European journal of pharmacology. 2010;645:185–191. doi: 10.1016/j.ejphar.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 191.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annual review of biochemistry. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 192.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 193.Chrivia JC, et al. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 194.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 195.Herzig S, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. The authors of this study use CREB knockout and dominant-negative mice to show that CREB increases hepatic gluconeogenesis and blood glucose through direct activation of PGC-1α expression. [DOI] [PubMed] [Google Scholar]
- 196.Herzig S, et al. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature. 2003;426:190–193. doi: 10.1038/nature02110. [DOI] [PubMed] [Google Scholar]
- 197.Lee JM, et al. AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB.CRTC2 complex by orphan nuclear receptor small heterodimer partner. The Journal of biological chemistry. 2010;285:32182–32191. doi: 10.1074/jbc.M110.134890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dentin R, et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. This study demonstrates that TORC2 plays a role in diabetic glucose homeostasis, since TORC2 levels are increased in diabetes and insulin inhibits the gluconeogenic gene program through phosphorylation and subsequent degradation of TORC2. [DOI] [PubMed] [Google Scholar]
- 199.Oh KJ, et al. TCF7L2 modulates glucose homeostasis by regulating CREB- and FoxO1-dependent transcriptional pathway in the liver. PLoS genetics. 2012;8:e1002986. doi: 10.1371/journal.pgen.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang EE, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nature medicine. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hammitzsch A, et al. CBP30, a selective CBP/p300 bromodomain inhibitor, suppresses human Th17 responses. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:10768–10773. doi: 10.1073/pnas.1501956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Best JL, et al. Identification of small-molecule antagonists that inhibit an activator: coactivator interaction. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17622–17627. doi: 10.1073/pnas.0406374101. An NMR-based screen is used in this work to identify small molecules that bind to CREB and disrupt its interaction with CBP, decreasing the cellular response to cAMP, showing that small molecules can inhibit cAMP signaling through interference of nuclear protein-protein interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang Y, et al. _targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3087–3092. doi: 10.1073/pnas.0914897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hirota T, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337:1094–1097. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang ND, et al. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. The authors use C/EBPα knockout mice to demonstrate that C/EBPα is needed for full activation of glycogen synthase and gluconeogenesis genes, as well as lipid accumulation in hepatocytes, establishing C/EBPα as a regulator of hepatic energy metabolism. [DOI] [PubMed] [Google Scholar]
- 206.Pedersen TA, et al. Distinct C/EBPalpha motifs regulate lipogenic and gluconeogenic gene expression in vivo. The EMBO journal. 2007;26:1081–1093. doi: 10.1038/sj.emboj.7601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Qiao L, MacDougald OA, Shao J. CCAAT/enhancer-binding protein alpha mediates induction of hepatic phosphoenolpyruvate carboxykinase by p38 mitogen-activated protein kinase. The Journal of biological chemistry. 2006;281:24390–24397. doi: 10.1074/jbc.M603038200. [DOI] [PubMed] [Google Scholar]
- 208.Arizmendi C, Liu S, Croniger C, Poli V, Friedman JE. The transcription factor CCAAT/enhancer-binding protein beta regulates gluconeogenesis and phosphoenolpyruvate carboxykinase (GTP) gene transcription during diabetes. The Journal of biological chemistry. 1999;274:13033–13040. doi: 10.1074/jbc.274.19.13033. [DOI] [PubMed] [Google Scholar]
- 209.Watanabe N, Osada H. Small molecules that _target phosphorylation dependent protein-protein interaction. Bioorganic & medicinal chemistry. 2016 doi: 10.1016/j.bmc.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 210.Solt LA, Kojetin DJ, Burris TP. The REV-ERBs and RORs: molecular links between circadian rhythms and lipid homeostasis. Future medicinal chemistry. 2011;3:623–638. doi: 10.4155/fmc.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yin L, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. This work reveals that the heme sensor and circadian clock component REV-ERBα decreases glucose output and gluconeogenic gene expression in liver cells. [DOI] [PubMed] [Google Scholar]
- 212.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 213.Chopra AR, et al. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke’s disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kadiri S, et al. The nuclear retinoid-related orphan receptor-alpha regulates adipose tissue glyceroneogenesis in addition to hepatic gluconeogenesis. American journal of physiology. Endocrinology and metabolism. 2015;309:E105–114. doi: 10.1152/ajpendo.00518.2014. [DOI] [PubMed] [Google Scholar]
- 215.Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug _targets. Nature reviews. Drug discovery. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Grant D, et al. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbalpha. ACS chemical biology. 2010;5:925–932. doi: 10.1021/cb100141y. [DOI] [PubMed] [Google Scholar]
- 217.Solt LA, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kumar N, et al. Identification of SR3335 (ML-176): a synthetic RORalpha selective inverse agonist. ACS chemical biology. 2011;6:218–222. doi: 10.1021/cb1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nature reviews. Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Louet JF, et al. The coactivator SRC-1 is an essential coordinator of hepatic glucose production. Cell metabolism. 2010;12:606–618. doi: 10.1016/j.cmet.2010.11.009. The authors find that Src-1 is induced by fasting and increases hepatic glucose production through activation of C/EBPα, which transactivates pyruvate carboxylase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang Y, et al. Small molecule inhibition of the steroid receptor coactivators, SRC-3 and SRC-1. Molecular endocrinology. 2011;25:2041–2053. doi: 10.1210/me.2011-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rousset S, et al. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl 1):S130–135. doi: 10.2337/diabetes.53.2007.s130. [DOI] [PubMed] [Google Scholar]
- 223.Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2011;7:205–212. doi: 10.1007/s13181-011-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Perry RJ, et al. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-_targeted mitochondrial uncoupler. Cell metabolism. 2013;18:740–748. doi: 10.1016/j.cmet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Perry RJ, Zhang D, Zhang XM, Boyer JL, Shulman GI. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science. 2015;347:1253–1256. doi: 10.1126/science.aaa0672. This study demonstrates that a controlled-release mitochondrial protonophore induces mild hepatic mitochondrial uncoupling and decreases insulin resistance and diabetes in rats without systemic toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989;38:550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- 227.Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Advances in nutrition. 2012;3:286–294. doi: 10.3945/an.112.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell metabolism. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. This review provides an overview of the regulatory mechanisms of hepatic glucose production, as well as a discussion of the controversies surrounding this topic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010;59:2697–2707. doi: 10.2337/db10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bandsma RH, et al. Acute inhibition of glucose-6-phosphate translocator activity leads to increased de novo lipogenesis and development of hepatic steatosis without affecting VLDL production in rats. Diabetes. 2001;50:2591. doi: 10.2337/diabetes.50.11.2591. [DOI] [PubMed] [Google Scholar]
- 231.Lei KJ, et al. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nature genetics. 1996;13:203–209. doi: 10.1038/ng0696-203. [DOI] [PubMed] [Google Scholar]
- 232.Clar J, et al. _targeted deletion of kidney glucose-6 phosphatase leads to nephropathy. Kidney international. 2014;86:747–756. doi: 10.1038/ki.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.van Schaftingen E, Gerin I. The glucose-6-phosphatase system. The Biochemical journal. 2002;362:513–532. doi: 10.1042/0264-6021:3620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wulffele MG, Kooy A, de Zeeuw D, Stehouwer CD, Gansevoort RT. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. Journal of internal medicine. 2004;256:1–14. doi: 10.1111/j.1365-2796.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 235.Befroy DE, et al. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nature medicine. 2014;20:98–102. doi: 10.1038/nm.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. Jama. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs. 1999;58(Suppl 1):31–39. doi: 10.2165/00003495-199958001-00009. discussion 75–82. [DOI] [PubMed] [Google Scholar]
- 238.El-Mir MY, et al. Dimethylbiguanide inhibits cell respiration via an indirect effect _targeted on the respiratory chain complex I. The Journal of biological chemistry. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 239.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. The Biochemical journal 348 Pt. 2000;3:607–614. [PMC free article] [PubMed] [Google Scholar]
- 240.Foretz M, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. The Journal of clinical investigation. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Miller RA, et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Madiraju AK, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fullerton MD, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nature medicine. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dornhorst A. Insulinotropic meglitinide analogues. Lancet. 2001;358:1709–1716. doi: 10.1016/S0140-6736(01)06715-0. [DOI] [PubMed] [Google Scholar]
- 246.Brown DX, Evans M. Choosing between GLP-1 Receptor Agonists and DPP-4 Inhibitors: A Pharmacological Perspective. Journal of nutrition and metabolism. 2012;2012:381713. doi: 10.1155/2012/381713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zinman B, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 248.Bailey T. Options for combination therapy in type 2 diabetes: comparison of the ADA/EASD position statement and AACE/ACE algorithm. The American journal of medicine. 2013;126:S10–20. doi: 10.1016/j.amjmed.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 249.Rojas LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetology & metabolic syndrome. 2013;5:6. doi: 10.1186/1758-5996-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Inzucchi SE, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 251.van Poelje PD, Potter SC, Erion MD. Fructose-1, 6-bisphosphatase inhibitors for reducing excessive endogenous glucose production in type 2 diabetes. Handbook of experimental pharmacology. 2011:279–301. doi: 10.1007/978-3-642-17214-4_12. [DOI] [PubMed] [Google Scholar]
- 252.Bantubungi K, et al. Cdkn2a/p16Ink4a regulates fasting-induced hepatic gluconeogenesis through the PKA-CREB-PGC1alpha pathway. Diabetes. 2014;63:3199–3209. doi: 10.2337/db13-1921. [DOI] [PubMed] [Google Scholar]