Abstract
Female sex is an independent risk factor for development of torsade de pointes (TdP) arrhythmias not only in congenital long QT syndromes but also in acquired long QT syndromes. Clinical and experimental evidences suggest that the gender differences may be due to, at least in part, gender differences in regulation of rate-corrected QT (QTC) interval between men and women. In adult women, both QTC interval and arrhythmic risks in TdP alter cyclically during menstrual cycle, suggesting a critical role of female sex hormones in cardiac repolarization process. These gender differences in fundamental cardiac electrophysiology result from variable ion channel expression and diverse sex hormonal regulation via long term genomic and acute non-genomic actions, and sex differences in drug responses and metabolisms. In particular, non-genomic actions of testosterone and progesterone on cardiac ion channels are likely to contribute to the gender differences in cardiac repolarization processes. This review summarizes current knowledge on sex hormonal regulation of cardiac ion channels which contribute to cardiac repolarization processes and its implication for gender differences in drug-induced long QT syndromes.
Keywords: sex hormones, cardiac repolarization, arrhythmias, gender difference
1. Introduction
Gender differences in electrocardiograms (ECGs) were reported for the first time in 1920(Bazett, 1920). In general, women have faster resting heart rates (Liu et al., 1989) and longer rate-corrected QT intervals in comparison with men (Di Diego et al., 2002; Fish et al., 2003; Hara et al., 1998; Merri et al., 1989; Pham et al., 2002; Xiao et al., 2006). Moreover, it is becoming clear that gender differences in cardiac electrophysiology play an important role in the prevalence of clinical arrhythmias.
It is widely accepted that women are more prone to develop drug-induced arrhythmia (specifically Torsade de Pointes, TdP) in association with prolongation of the QT interval, which corresponds to the duration of the ventricular action potential(Abi-Gerges et al., 2004). Although the underlying reasons for the gender disparity in incidence of TdP have yet to be completely clarified, clinical evidences imply that sex steroid hormones appear to play important roles in gender differences by affecting the cardiac repolarization process of action potential(Bai et al., 2005; Furukawa et al., 2007; Korte et al., 2005; Nakagawa et al., 2005; Verkerk et al., 2005; Xiao et al., 2006). Shortening of QT interval after puberty in men have been cited as an evidence that male hormone is involved in cardiac repolarization process (Rautaharju et al., 1992). Shorter QT intervals in women with virilization than normal women and castrated men (Bidoggia et al., 2000) imply that androgens accelerate cardiac repolarization processes and shorten action potential durations (APD), which was demonstrated in rabbit models (Pham et al., 2002). In terms of female hormones, it has been known that dynamic changes in levels of female sex hormones during women’s menstrual cycle have cyclical effects on QT intervals, implying that serum concentrations of female sex hormones impact cardiac ion channel functions.
Sex steroid hormones have been traditionally considered to act via the regulation of transcriptional processes (Mangelsdorf et al., 1995). In addition to genomic regulation, accumulating evidences, however, show that the sex hormones can also exhibit rapid effects involving intracellular signaling, and are therefore referred to as “non-genomic regulation”(Baron et al., 2004; Furukawa et al., 2007). Non-genomic acute effects of sex steroid hormones take place outside the cell nucleus via the activation of specific signaling pathways that include eNOS and MAP kinase. In addition to genomic regulation, we recently reported that physiological levels of sex hormones can acutely modify cardiac repolarization by regulating cardiac ion channels via either a non-genomic pathway involving hormone receptors or in a receptor-independent fashion. These non-genomic actions likely contribute to the gender differences in QTC intervals and susceptibility of TdP. A computational approach revealed a significant impact of non-genomic regulations of sex hormones on susceptibility of long QT linked arrhythmias(Yang et al., 2010a; Yang et al., 2010b).
The main goal of this review article is to provide an overview of how cardiac repolarization is regulated by sex hormones, then to discuss the implication to occurrence of drug-induced long QT syndrome.
2. Gender differences in drug-induced long QT syndrome
It is now apparent that TdP, a life-threatening ventricular tachyarrhythmia can be induced by common medications which prolong duration of the cardiac action potential (Drici et al., 1998; Hashimoto, 2007; Hreiche et al., 2008; James et al., 2007; Lehmann et al., 1996; Makkar et al., 1993; Tamargo, 2000). Acquired long QT syndrome (LQTS) can be associated with drug-induced prolonged QTC intervals and arrhythmia, and results from delay of cardiac repolarization caused mostly by inhibition of the human ether-a-go-go-related gene (hERG) channel, which conducts the rapid component of the delayed rectifier K+ current (IKr). Thus, regulatory agencies require pre-clinical hERG inhibition data for any drug candidate (Abriel et al., 2004; Fermini et al., 2003; Satoh et al., 2000; Takahara et al., 2007). However, the predictability of clinical arrhythmias with hERG test is often debated because of too many false positives. Actually, the incidence of drug-induced arrhythmia is also affected by other risk factors such as other channel inhibition, gender (Drici et al., 1998; Lehmann et al., 1996; Makkar et al., 1993) or/and sympathetic nervous system activity (Kurokawa, 2007; Marx et al., 2002). Thus, unraveling of the molecular basis of effects of such risk factors may be beneficial for improving predictability of this lethal arrhythmia caused by medication.
It has been shown that female sex is an independent risk factor for development of drug-induced TdP in patients with 65–75% of cases occurring in women (Drici et al., 1998; Lehmann et al., 1996; Locati et al., 1998; Makkar et al., 1993). The higher risks in women have been thought to be associated with the 20-ms prolonged baseline QTC intervals in women in comparison with those in men(Bazett, 1920). The gender differences in QTC intervals appear to correlate with age-dependent changes in serum levels of sex hormones. There is no statistical difference in the QTC interval between men and women before puberty (Merri et al., 1989; Rautaharju et al., 1992; Stramba-Badiale et al., 1995). As sex hormone levels increase during puberty, QTC intervals in men are shortened, leaving adult women with longer QTC intervals than adult men. The QTC interval in men then gradually increases until the age of ~60 years when the intervals approach that of women (Merri et al., 1989; Rautaharju et al., 1992; Stramba-Badiale et al., 1995). The age-dependent changes in QTC intervals in men imply the involvement of androgens in these gender differences. Although we had reported that testosterone shortens APD in guinea pig by decreasing the L-type calcium current (ICa,L; an inward depolarizing current) and increasing slow delayed rectifier potassium current (IKs; an outward repolarizing current) (Bai et al., 2005), recent simulation study showed that the early repolarization changes in men were most influenced by the effect of testosterone on calcium currents (Vicente et al., 2014).
Female sex hormones are also involved in the gender differences both in QTC intervals and in the susceptibility to TdP, although the situation is more complex than that for androgens. During the menstrual cycle and pregnancy in females, there are dynamic fluctuations in QT interval and TdP risk which may correlate with changes in serum levels of ovarian steroids (Nakagawa et al., 2006). Several studies have evaluated the potential impact of hormone replacement therapy (HRT) on QTC intervals in postmenopausal women (Abi-Gerges et al., 2004; Kadish et al., 2004). Although conflicting findings exist regarding HRT, these clinical evidences imply that the dynamic changes in levels of female sex hormones, progesterone and estrogen, have cyclical effects on action potential duration. The effects of female hormones on ventricular repolarization will be discussed in the following sections.
3. Sex hormonal regulations of cardiac ion channels
The gender differences in drug-induced long QT syndrome is partly based on the electrical differences resulting from expression of various ion channels. Analysis with human male and female showed decrease expression for several repolarizing channels including hERG (Gaborit et al., 2010). Sex hormones bind to sex hormone receptors, translocate into nucleus, and regulate the transcription of _target genes. There are some reports the transcriptional regulation of cardiac ion channels by sex hormones. In cardiomyocytes, testosterone increases expression of the L-type Ca2+ channels and the Na+-Ca2+ exchanger in mRNA (Golden et al., 2004). Androgen enhances expression of IKur and KV1.5 resulting in short ventricular repolarization (Brouillette et al., 2005). The chronic effects of testosterone and E2 include the transcriptional regulation of some K+ channels, but not the hERG channel (Drici et al., 1996; Song et al., 2001). In addition to conventional genomic pathway of sex hormone, E2 has also reported to augment membrane trafficking of hERG by enhancing interaction to heat shock proteins in non-genomic pathway (Anneken et al., 2016). Chronic application of progesterone in hearts did not change protein expression of L-type Ca2+ channels (Helguera et al., 2002) and the Kv4.3 channel (Song et al., 2001).
Several reports have shown that sex steroid hormones rapidly inhibit various cardiac ion currents, including L-type Ca2+ currents (ICa,L), T-type Ca2+ currents (ICa,T), slowly- and rapidly-activating delayed rectifier K+ currents (IKs and IKr), transient outward current (Ito), and inwardly rectifying K+ current (IK1) in isolated cardiomyocytes(Abi-Gerges et al., 2004). However, to elicit these rapid inhibitions, estrogen and androgens were applied at levels (several μM) higher than their physiological levels. At these concentrations, sex steroids exhibit a non-specific binding to various molecules, and the resulting signaling pathways are also not known.
Reports of the effects of sex hormones within the physiological range are limited. We found in guinea pig ventricular myocytes that acute applications of physiological serum levels of testosterone and progesterone suppress a depolarizing current, ICa,L, and enhance a repolarizing current, IKs, resulting in shortening of the duration of ventricular action potentials. Thus, testosterone and progesterone shortens the QTC interval and likely exhibit anti-arrhythmic effects (Bai et al., 2005; Nakamura et al., 2007). The regulation of ICa,L and IKs occurs within 10 to 15 min via a non-genomic pathway involving sequential activation of c-Src, PI3-kinase, Akt, and eNOS and resultant release of nitric oxide (NO) (Bai et al., 2005; Nakamura et al., 2007). Pharmacological assays using inhibitors of the signaling molecules and sucrose density gradient fractionation experiments strongly suggest that the non-genomic pathway occurs in the caveolae/lipid raft domain (Nakamura et al., 2007). In contrast to progesterone and testosterone, 17β-estradiol, a potent estrogen, shortens the duration of ventricular action potentials only at levels higher than physiological serum concentrations, suggesting a minimal impact on the regulation of ICa,L and IKs (Bai et al., 2005; Kurokawa et al., 2008). The difference between progesterone and estrogen likely contributes differently to dynamic changes of the action potential duration during the menstrual cycle in females.
4. Non-genomic action of progesterone
Progesterone has robust non-genomic effects on cardiac ion channels. Progesterone at maximal effective concentration enhances IKs by ~140%, and suppresses ICa,L by ~60%. Both of these effects were abolished by NO scavenger or inhibitors of signaling molecules in the non-genomic pathway. The suppression of ICa,L by progesterone was cGMP-dependent, as described previously (Fischmeister et al., 2005), while the enhancement of IKs was independent of the cGMP-soluble guanylate cyclase (sGC) pathway, and may involve protein s-nitrosylation(Asada et al., 2009). Since antagonistic effects of cGMP on cAMP-stimulated ICa,L have been demonstrated, it is tempting to consider the possibility of crosstalk with signaling mediated by cAMP. Actually, sympathetic nervous system (SNS) stimulation, a critical triggering factor for TdP in LQTS (Kass et al., 2003), altered the properties of the _target ion channels. (Figure 1). Under basal conditions without cAMP stimulation, progesterone enhanced IKs in a dose-dependent fashion (EC50 = 2.7 nM, the progesterone level in the luteal phase, ~40.6 nM) without modifying ICa,L in this dose range(Janse de Jonge et al., 2001). In the presence of SNS stimulation, progesterone partially suppressed ICa,L in a dose-dependent fashion (IC50 = 29.9 nM), while 100 nM progesterone did not significantly affect IKs. Because the reported progesterone level in women is ~2.5 nM in the follicular phase and ~40.6 nM in the luteal phase (Janse de Jonge et al., 2001), cyclical fluctuation of serum progesterone during the menstrual cycle may affect modulation of ICa,L and IKs.
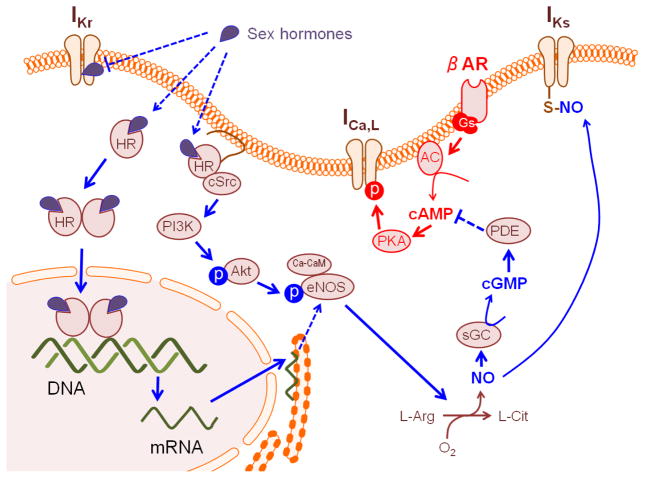
Figure 1.
Scheme for mechanism underlying sex hormonal regulation of the L-type Ca2+ (ICa,L) channel, the rapid delayed rectifier K+ (IKr) channel and the slow delayed rectifier K+ (IKs) channel. HR; hormone receptor, βAR; beta-adrenergic receptor, AC; adenylate cyclase, PDE; phosphodiesterase, L-Arg; L-arginine, L-Cit; L-citrulline, sGC; soluble guanylyl cyclase. βAR stimulation involves in modulation of ICa,L through a non-genomic pathway of progesterone receptor(Nakamura et al., 2007).
5. Estrogen
Estrogen has a number of cardiovascular effects. There is much evidence to suggest that estrogen may reduce the risk of arrhythmias indirectly by protecting against cardiac ischemic injuries as a consequence of vasodilation (Murphy et al., 2008). Although clinical impacts of estrogen on drug-induced LQTS are still debated, chronic application of estrogen has some impact in some cases of drug-induced LQTS (Abi-Gerges et al., 2004; Drici et al., 1998; Hara et al., 1998). Chronic treatment with 17β-estradiol (E2) enhanced action potential duration prolongation in rabbit papillary muscle and the incidence and magnitude of early afterdepolarizations (EADs) induced by E4031, a hERG blocker (Hara et al., 1998). Because the E2 treatment did not affect baseline electrocardiographic characteristics, chronic E2 treatment appears to reduce the repolarization reserve (Hara et al., 1998) which indicates the existence of redundancy of repolarizing currents in cardiomyocytes. But, it is difficult to clearly discuss the relative impact of estrogen-induced regulation on cardiac repolarization because of the existence of several unknown transcriptional _targets and wide interspecies variation (Drici et al., 1996; Fulop et al., 2006).
There are conflicting evidences regarding the effects of estrogen- or hormone-replacement therapy (ERT or HRT) on QTC intervals in post-menopausal women. Although it has been reported that ERT did not alter QTC intervals(Abi-Gerges et al., 2004), a large-scale clinical study of post-menopausal women revealed very slight (a few msecs), but significant, QTC prolongation with ERT alone(Kadish et al., 2004). This study suggests that exogenous estrogen moderately delays cardiac repolarization in a reversible fashion, suggesting the existence of acute effects of estrogen(Rodriguez et al., 2001). Consistent with impacts of ERT on ECG in a large-scale clinical study, our recent data from guinea pig ventricular myocytes revealed that physiological concentrations of E2 acutely delayed cardiac repolarization, resulting in a slight prolongation of the QTC interval and action potential duration (APD)(Kurokawa et al., 2008). Circulating physiological concentrations of E2 vary from 0.1 to 1 nM during the menstrual cycle (<0.1 nM in men), and rise to as high as several hundred nM only during pregnancy (Hulot et al., 2003; James et al., 2007; Nakagawa et al., 2006; Rodriguez et al., 2001).
E2 has dual effects on electric function in guinea pig ventricles. Physiological concentrations of E2 inhibit IKr (Kd = 1.3 nM) and prolong QTC interval and APD, while higher (non-physiological) concentrations of E2 yielded not only IKr inhibition, but also non-genomic regulation enhancing IKs (Kd = 39.4 nM) and suppressing ICa,L (Kd = 29.5 nM) as described above (Kurokawa et al., 2008). The magnitude of E2-induced IKr suppression was statistically significant, but relatively small (<30%) (Kurokawa et al., 2008). This is consistent with the evidences implying that estrogen has less clear impacts on baseline QTC intervals (Hulot et al., 2003; Nakagawa et al., 2006; Rodriguez et al., 2001). Another estrogen, estrone 3-sulfate at physiological concentrations in both women and men at any age has been reported to suppress hERG currents to about 80% (Kakusaka et al., 2009).
E2 suppresses IKr in a receptor-independent manner. A mutagenesis study of common drug-binding sites of the hERG channel (Clancy et al., 2003; Fernandez et al., 2004; Sanguinetti et al., 2005) revealed that aromaticity of Phe656 is important for E2-induced hERG suppression, suggesting that the aromatic-centroid of E2, which exists only in estrogen and not in other sex steroids, may be responsible for modulation of the hERG channel (Kurokawa et al., 2008) (Figure 2A). Although the mechanism responsible for it has not been clarified, E2 enhances that E4031-induced hERG suppression (Figure 2B), in line with the increase in ibutilide-induced QTC prolongation in the late follicular phase (Rodriguez et al., 2001), which could underlie the enhanced susceptibility of women to acquired LQTS (Drici et al., 1998; James et al., 2007; Lehmann et al., 1996; Makkar et al., 1993).
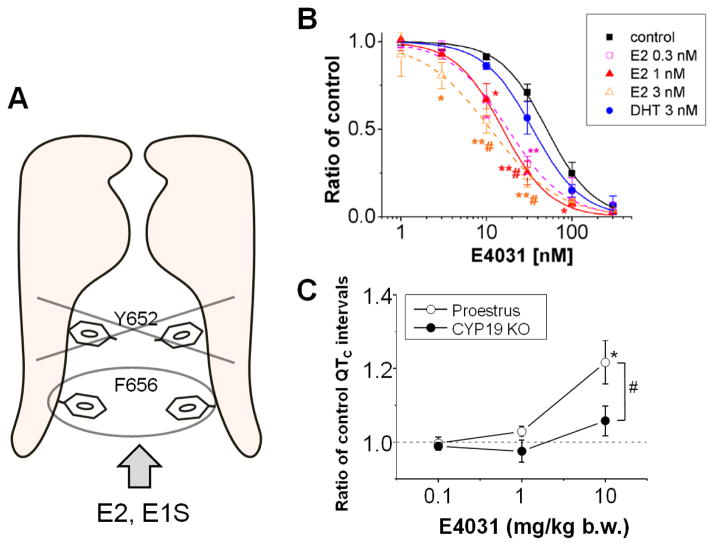
Figure 2.
Site specific effect of estrogen alters sensitivity of a selective HERG blocker, E4031. A. A scheme shows that Phe656 (F656) but not Tyr652 (Y652), a common drug-binding site for the hERG, is important for the suppression by some estrogens (E2 and E1S)(Kakusaka et al., 2009; Kurokawa et al., 2008). B. E2 but not DHT (dihydrotestosterone) shifted concentration-dependent curves of HERG inhibition by E4031.. HERG currents were recorded from HEK293 cells overexpressing the hERG channel. E2 at 0.3, 1, 3 nM increased the E4031-induced fractional inhibition (*P<0.05 vs. control, and vs. DHT), while DHT did not (n.s. vs. control)(Kurokawa et al., 2008). C. Estrogen-null in CYP19 KO mice reduced the E-4031-induced QTc prolongation. Prolongation of QTc intervals in response to the cumulative administration of E-4031 were compared between late-proestrus female mice (open circles) and aromatase knockout mice (CYP19 KO, closed circles). Modified from(Kurokawa et al., 2015).
Recently, employment of an aromatase knockout mouse as an in vivo estrogen-null model supports the effects of estrogen on cardiac electrophysiology(Kurokawa et al., 2015). The knockout mice were generated by _targeted disruption of the CYP19 gene coding aromatase cytochrome P450 (Honda et al., 1998). The effects of E-4031 on electrocardiogram parameters were compared between wild-type mice (C57/BL6J) and the aromatase knockout mice. The ablation of circulating estrogens blunted the effects of E-4031 on heart rate and QT interval in mice under a denervation condition. The result provides in vivo proof of principle and demonstrates that endogenous estrogens increase the sensitivity of cardiac repolarization to hERG block by E-4031 (Figure 2C)..
6. Computational modeling and simulation approaches
Based on clinical and experimental studies suggesting that differences in arrhythmia vulnerability may stem from sex steroid hormones, mathematical modeling and simulation approaches have been developed to try to reveal plausible mechanisms of sex steroid hormone linked arrhythmia vulnerability (Yang et al., 2010a; Yang et al., 2011)(Roberts et al., 2012)(Grace et al., 2012).
The sex steroid hormones estrogen and progesterone and their acute interactions with multiple subcellular _targets in the heart have been incorporated into computationally based models of cardiac ion channels to predict how they alter the emergent electrical activity of guinea pig cells and tissues (Faber et al., 2000; Yang et al., 2010b). Models allow for simulation of the effects of individual components or components in combination and so have been used to tease out the individual contributions of estrogen, progesterone and testosterone on cardiac electrical behavior and then make predictions about their effects in combination and in the presence of drugs. The guinea pig model simulations reproduced observed fluctuations of cardiac repolarization during the menstrual cycle in females, and predicted protective effects of progesterone against rhythm disturbance in a cellular and a tissue model of congenital and drug-induced LQTS(Nakamura et al., 2007). These findings (Nakamura et al., 2007) suggested that non-genomic regulation of cardiac ion channels by progesterone may influence fluctuation of baseline QTC during the menstrual cycle in females, consistent with previous clinical reports (Hulot et al., 2003; Nakagawa et al., 2006; Rodriguez et al., 2001). Non-genomic effects of progesterone on ICa,L and IKs depict well how mathematical models of cardiac electrophysiology help to understand the mechanism of development of cardiac arrhythmias.
The computational models in guinea pig virtual cells have also shown that simulation of sex steroid hormones during the menstrual cycle in females can reproduce observed fluctuations of QT intervals as recorded on the ECG. The effects of testosterone on ECG parameters were also reproducibly simulated. Modeling predictions suggested that testosterone and progesterone are protective against drug-induced arrhythmias, while estrogen likely exacerbates the breakdown of normal cardiac electrical activity in the presence of QT-prolonging drugs (Yang et al., 2010b).
Studies utilizing complementary computational and experimental approaches have also been used to inform human in silico models to determine if sex based differences in physiologically relevant concentrations of sex steroid hormones will exacerbate or protect against initiation of human self-sustaining reentrant arrhythmias, a clinically significant precedent event to lethal arrhythmias that has been observed with significantly higher incidence in women in the setting of acquired LQTS (Yang et al., 2012).
Mathematical models have been developed to represent in silico male and female ventricular human heart cells by incorporating experimentally determined genomic differences and effects of sex steroid hormones into the O’Hara-Rudy human virtual myocyte model (O’Hara et al., 2011) (Yang et al., 2012). The model incorporated genome-scale measurements of differences between human males and females. For example, human females have been shown to have lower expression of genes encoding key cardiac repolarizing potassium currents and connexin43, the primary ventricular gap-junction subunit. Human males and females also have distinct sex steroid hormones. The “male” and “female” model cells and tissues then were used to predict how various sex-based differences underlie arrhythmia risk. Genomic-based differences in ion channel expression were alone sufficient to determine longer female cardiac APDs in both epicardial and endocardial cells compared to males. Addition of sex steroid hormones exacerbated these differences, as testosterone was predicted to further shorten APD, while estrogen and progesterone application resulted in disparate effects on APD.
Computational modeling studies predict that during the menstrual follicular phase (prior to ovulation), QT interval is longer than in the luteal phase (following ovulation) when progesterone (Pg) is increased. Moreover, susceptibility to drug-induced arrhythmias was predicted to be most likely in the late follicular phase where estrogen (E2) level is the highest (Yang et al., 2012).
Model predictions suggested that males have redundant protective mechanisms including testosterone and more prominent connexin43, which may contribute to shorter rate corrected QT intervals compared to females. With application of 35 nM DHT, the model simulations suggested that a reentrant wave cannot be sustained, even in the presence of a hERG blocking drug (Yang et al., 2012).
The increase in connexin43 was predicted to play a key factor for preventing sustained reentry in males. Indeed, we made some _targeted swaps of model components to show this directly (Yang et al., 2012). When female hormones and an IKr blocker was added to the “genetic” male model and tested for vulnerability to reentry, the male was still protected. Although the addition of drug and female hormones had the expected effect to increase APD, the faster male conduction velocity resulted in a rapid collision of the wave front with the wave tail after a single rotation. Similar behavior was observed when the genetic female model with female hormones corresponding to the late follicular phase was combined with the IKr blocker, but male connexin 43 level was swapped into the model alone. These simulations suggests that sex-based differences in conduction play an important role in arrhythmia vulnerability.
In the future, modeling and simulation approaches should be carried out to examine mechanisms of arrhythmic events associated with acquired and inherited LQTs during phases where progesterone is high, especially in the setting of sympathetic stimulation, a major factor in triggering TdP.
7. Summary
We have summarized here recent progress in examination of gender differences in susceptibility to arrhythmias including drug-induced LQTS (Figure 1). In addition to well-characterized genomic effects of ovarian steroids, non-genomic effects mediated via the progesterone receptor(Nakamura et al., 2007) and receptor-independent regulation by estrogen (Kurokawa et al., 2008) have recently been introduced as novel possible causes of the higher susceptibility to drug-induced LQTS in women. The acute effects of female hormones could be taken into account in assessing the risk of drug-induced QT prolongation especially in women.
Major remaining issues that need attention could be collection of human data and integration of emergent effects of sex hormones on multiple intersecting processes. Employments of human iPS cell-derived cardiomyocytes and mathematical simulation would be possible solutions to translate knowledge of these basic researches into toxicological assessments.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Gerges N, Philp K, Pollard C, Wakefield I, Hammond TG, Valentin JP. Sex differences in ventricular repolarization: from cardiac electrophysiology to Torsades de Pointes. Fundam Clin Pharmacol. 2004;18(2):139–151. doi: 10.1111/j.1472-8206.2004.00230.x. [DOI] [PubMed] [Google Scholar]
- Abriel H, Schlapfer J, Keller DI, Gavillet B, Buclin T, Biollaz J, et al. Molecular and clinical determinants of drug-induced long QT syndrome: an iatrogenic channelopathy. Swiss Med Wkly. 2004;134(47–48):685–694. doi: 10.4414/smw.2004.10532. [DOI] [PubMed] [Google Scholar]
- Anneken L, Baumann S, Vigneault P, Biliczki P, Friedrich C, Xiao L, et al. Estradiol regulates human QT-interval: acceleration of cardiac repolarization by enhanced KCNH2 membrane trafficking. European heart journal. 2016;37(7):640–650. doi: 10.1093/eurheartj/ehv371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K, Kurokawa J, Furukawa T. Redox- and Calmodulin-dependent S-Nitrosylation of the KCNQ1 Channel. The Journal of biological chemistry. 2009;284(9):6014–6020. doi: 10.1074/jbc.M807158200. [DOI] [PubMed] [Google Scholar]
- Bai CX, Kurokawa J, Tamagawa M, Nakaya H, Furukawa T. Nontranscriptional regulation of cardiac repolarization currents by testosterone. Circulation. 2005;112(12):1701–1710. doi: 10.1161/CIRCULATIONAHA.104.523217. [DOI] [PubMed] [Google Scholar]
- Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, et al. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. The Journal of biological chemistry. 2004;279(15):14579–14586. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- Bidoggia H, Maciel JP, Capalozza N, Mosca S, Blaksley EJ, Valverde E, et al. Sex differences on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. American heart journal. 2000;140(4):678–683. doi: 10.1067/mhj.2000.109918. [DOI] [PubMed] [Google Scholar]
- Brouillette J, Rivard K, Lizotte E, Fiset C. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovascular research. 2005;65(1):148–157. doi: 10.1016/j.cardiores.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Clancy CE, Kurokawa J, Tateyama M, Wehrens XH, Kass RS. K+ channel structure-activity relationships and mechanisms of drug-induced QT prolongation. Annu Rev Pharmacol Toxicol. 2003;43:441–461. doi: 10.1146/annurev.pharmtox.43.100901.140245. [DOI] [PubMed] [Google Scholar]
- Di Diego JM, Cordeiro JM, Goodrow RJ, Fish JM, Zygmunt AC, Perez GJ, et al. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation. 2002;106(15):2004–2011. doi: 10.1161/01.cir.0000032002.22105.7a. [DOI] [PubMed] [Google Scholar]
- Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex Hormones Prolong the QT Interval and Downregulate Potassium Channel Expression in the Rabbit Heart. Circulation. 1996;94(6):1471–1474. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- Drici MD, Knollmann BC, Wang WX, Woosley RL. Cardiac actions of erythromycin: influence of female sex. JAMA. 1998;280(20):1774–1776. doi: 10.1001/jama.280.20.1774. [DOI] [PubMed] [Google Scholar]
- Faber GM, Rudy Y. Action potential and contractility changes in [Na(+)](i) overloaded cardiac myocytes: a simulation study. Biophys J. 2000;78(5):2392–2404. doi: 10.1016/S0006-3495(00)76783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermini B, Fossa AA. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov. 2003;2(6):439–447. doi: 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- Fernandez D, Ghanta A, Kauffman GW, Sanguinetti MC. Physicochemical features of the HERG channel drug binding site. The Journal of biological chemistry. 2004;279(11):10120–10127. doi: 10.1074/jbc.M310683200. [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Castro L, Abi-Gerges A, Rochais F, Vandecasteele G. Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels. Comp Biochem Physiol A Mol Integr Physiol. 2005;142(2):136–143. doi: 10.1016/j.cbpb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Fish JM, Antzelevitch C. Cellular and ionic basis for the sex-related difference in the manifestation of the Brugada syndrome and progressive conduction disease phenotypes. J Electrocardiol. 2003;36(Suppl):173–179. doi: 10.1016/j.jelectrocard.2003.09.054. [DOI] [PubMed] [Google Scholar]
- Fulop L, Banyasz T, Szabo G, Toth IB, Biro T, Lorincz I, et al. Effects of sex hormones on ECG parameters and expression of cardiac ion channels in dogs. Acta Physiol (Oxf) 2006;188(3–4):163–171. doi: 10.1111/j.1748-1716.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Kurokawa J. Regulation of cardiac ion channels via non-genomic action of sex steroid hormones: implication for the gender difference in cardiac arrhythmias. Pharmacol Ther. 2007;115(1):106–115. doi: 10.1016/j.pharmthera.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Gaborit N, Varro A, Le Bouter S, Szuts V, Escande D, Nattel S, et al. Gender-related differences in ion-channel and transporter subunit expression in non-diseased human hearts. Journal of molecular and cellular cardiology. 2010;49(4):639–646. doi: 10.1016/j.yjmcc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Golden KL, Marsh JD, Jiang Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2004;36(4):197–202. doi: 10.1055/s-2004-814445. [DOI] [PubMed] [Google Scholar]
- Grace AA, Roden DM. Systems biology and cardiac arrhythmias. Lancet. 2012;380(9852):1498–1508. doi: 10.1016/S0140-6736(12)61462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Danilo P, Jr, Rosen MR. Effects of gonadal steroids on ventricular repolarization and on the response to E4031. J Pharmacol Exp Ther. 1998;285(3):1068–1072. [PubMed] [Google Scholar]
- Hashimoto K. Arrhythmia models for drug research: classification of antiarrhythmic drugs. J Pharmacol Sci. 2007;103(4):333–346. doi: 10.1254/jphs.crj06013x. [DOI] [PubMed] [Google Scholar]
- Helguera G, Olcese R, Song M, Toro L, Stefani E. Tissue-specific regulation of Ca(2+) channel protein expression by sex hormones. Biochimica et biophysica acta. 2002;1569(1–3):59–66. doi: 10.1016/s0304-4165(01)00234-3. [DOI] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun. 1998;252(2):445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- Hreiche R, Morissette P, Turgeon J. Drug-induced long QT syndrome in women: review of current evidence and remaining gaps. Gend Med. 2008;5(2):124–135. doi: 10.1016/j.genm.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Hulot J-S, Demolis J-L, Riviere R, Strabach S, Christin-Maitre S, Funck-Brentano C. Influence of endogenous oestrogens on QT interval duration. European heart journal. 2003;24(18):1663–1667. doi: 10.1016/s0195-668x(03)00436-6. [DOI] [PubMed] [Google Scholar]
- James AF, Choisy SC, Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol. 2007;94(3):265–319. doi: 10.1016/j.pbiomolbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Janse de Jonge XA, Boot CR, Thom JM, Ruell PA, Thompson MW. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J Physiol. 2001;530(Pt 1):161–166. doi: 10.1111/j.1469-7793.2001.0161m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadish AH, Greenland P, Limacher MC, Frishman WH, Daugherty SA, Schwartz JB. Estrogen and progestin use and the QT interval in postmenopausal women. Ann Noninvasive Electrocardiol. 2004;9(4):366–374. doi: 10.1111/j.1542-474X.2004.94580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakusaka S, Asayama M, Kaihara A, Sasano T, Suzuki T, Kurokawa J, et al. A receptor-independent effect of estrone sulfate on the HERG channel. J Pharmacol Sci. 2009;109(1):152–156. doi: 10.1254/jphs.08257sc. [DOI] [PubMed] [Google Scholar]
- Kass RS, Kurokawa J, Marx SO, Marks AR. Leucine/isoleucine zipper coordination of ion channel macromolecular signaling complexes in the heart. Roles in inherited arrhythmias. Trends Cardiovasc Med. 2003;13(2):52–56. doi: 10.1016/s1050-1738(02)00211-6. [DOI] [PubMed] [Google Scholar]
- Korte T, Fuchs M, Arkudas A, Geertz S, Meyer R, Gardiwal A, et al. Female mice lacking estrogen receptor beta display prolonged ventricular repolarization and reduced ventricular automaticity after myocardial infarction. Circulation. 2005;111(18):2282–2290. doi: 10.1161/01.CIR.0000164262.08004.BB. [DOI] [PubMed] [Google Scholar]
- Kurokawa J. Compartmentalized regulations of ion channels in the heart. Biol Pharm Bull. 2007;30(12):2231–2237. doi: 10.1248/bpb.30.2231. [DOI] [PubMed] [Google Scholar]
- Kurokawa J, Sasano T, Kodama M, Li M, Ebana Y, Harada N, et al. Aromatase knockout mice reveal an impact of estrogen on drug-induced alternation of murine electrocardiography parameters. J Toxicol Sci. 2015;40(3):339–348. doi: 10.2131/jts.40.339. [DOI] [PubMed] [Google Scholar]
- Kurokawa J, Tamagawa M, Harada N, Honda S, Bai CX, Nakaya H, et al. Acute effects of oestrogen on the guinea pig and human IKr channels and drug-induced prolongation of cardiac repolarization. J Physiol. 2008;586(Pt 12):2961–2973. doi: 10.1113/jphysiol.2007.150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann MH, Hardy S, Archibald D, quart B, MacNeil DJ. Sex difference in risk of torsade de pointes with d,l-sotalol. Circulation. 1996;94(10):2535–2541. doi: 10.1161/01.cir.94.10.2535. [DOI] [PubMed] [Google Scholar]
- Liu K, Ballew C, Jacobs DR, Jr, Sidney S, Savage PJ, Dyer A, et al. Ethnic differences in blood pressure, pulse rate, and related characteristics in young adults. The CARDIA study. Hypertension. 1989;14(2):218–226. doi: 10.1161/01.hyp.14.2.218. [DOI] [PubMed] [Google Scholar]
- Locati EH, Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Lehmann MH, et al. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the International LQTS Registry. Circulation. 1998;97(22):2237–2244. doi: 10.1161/01.cir.97.22.2237. [DOI] [PubMed] [Google Scholar]
- Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270(21):2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295(5554):496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- Merri M, Benhorin J, Alberti M, Locati E, Moss AJ. Electrocardiographic quantitation of ventricular repolarization. Circulation. 1989;80(5):1301–1308. doi: 10.1161/01.cir.80.5.1301. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88(2):581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Ooie T, Ou B, Ichinose M, Takahashi N, Hara M, et al. Gender differences in autonomic modulation of ventricular repolarization in humans. J Cardiovasc Electrophysiol. 2005;16(3):278–284. doi: 10.1046/j.1540-8167.2005.40455.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Ooie T, Takahashi N, Taniguchi Y, Anan F, Yonemochi H, et al. Influence of Menstrual Cycle on QT Interval Dynamics. Pacing and Clinical Electrophysiology. 2006;29(6):607–613. doi: 10.1111/j.1540-8159.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kurokawa J, Bai CX, Asada K, Xu J, Oren RV, et al. Progesterone regulates cardiac repolarization through a nongenomic pathway: an in vitro patch-clamp and computational modeling study. Circulation. 2007;116(25):2913–2922. doi: 10.1161/CIRCULATIONAHA.107.702407. [DOI] [PubMed] [Google Scholar]
- O’Hara T, Virag L, Varro A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol. 2011;7(5):e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TV, Robinson RB, Danilo P, Jr, Rosen MR. Effects of gonadal steroids on gender-related differences in transmural dispersion of L-type calcium current. Cardiovascular research. 2002;53(3):752–762. doi: 10.1016/s0008-6363(01)00449-7. [DOI] [PubMed] [Google Scholar]
- Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8(7):690–695. [PubMed] [Google Scholar]
- Roberts BN, Yang PC, Behrens SB, Moreno JD, Clancy CE. Computational approaches to understand cardiac electrophysiology and arrhythmias. Am J Physiol Heart Circ Physiol. 2012;303(7):H766–783. doi: 10.1152/ajpheart.01081.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. Jama. 2001;285(10):1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Mitcheson JS. Predicting drug-hERG channel interactions that cause acquired long QT syndrome. Trends Pharmacol Sci. 2005;26(3):119–124. doi: 10.1016/j.tips.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Sugiyama A, Tamura K, Hashimoto K. Effects of mexiletine on the canine cardiovascular system complicating cisapride overdose: potential utility of mexiletine for the treatment of drug-induced long QT syndrome. Jpn J Pharmacol. 2000;83(4):327–334. doi: 10.1254/jjp.83.327. [DOI] [PubMed] [Google Scholar]
- Song M, Helguera G, Eghbali M, Zhu N, Zarei MM, Olcese R, et al. Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. The Journal of biological chemistry. 2001;276(34):31883–31890. doi: 10.1074/jbc.M101058200. [DOI] [PubMed] [Google Scholar]
- Stramba-Badiale M, Spagnolo D, Bosi G, Schwartz PJ. Are gender differences in QTc present at birth? MISNES Investigators. Multicenter Italian Study on Neonatal Electrocardiography and Sudden Infant Death Syndrome. Am J Cardiol. 1995;75(17):1277–1278. [PubMed] [Google Scholar]
- Takahara A, Nakamura H, Nouchi H, Tamura T, Tanaka T, Shimada H, et al. Analysis of arrhythmogenic profile in a canine model of chronic atrioventricular block by comparing in vitro effects of the class III antiarrhythmic drug nifekalant on the ventricular action potential indices between normal heart and atrioventricular block heart. J Pharmacol Sci. 2007;103(2):181–188. doi: 10.1254/jphs.fp0061077. [DOI] [PubMed] [Google Scholar]
- Tamargo J. Drug-induced torsade de pointes: from molecular biology to bedside. Jpn J Pharmacol. 2000;83(1):1–19. doi: 10.1254/jjp.83.1. [DOI] [PubMed] [Google Scholar]
- Verkerk AO, Wilders R, Veldkamp MW, de Geringel W, Kirkels JH, Tan HL. Gender disparities in cardiac cellular electrophysiology and arrhythmia susceptibility in human failing ventricular myocytes. Int Heart J. 2005;46(6):1105–1118. doi: 10.1536/ihj.46.1105. [DOI] [PubMed] [Google Scholar]
- Vicente J, Johannesen L, Galeotti L, Strauss DG. Mechanisms of sex and age differences in ventricular repolarization in humans. American heart journal. 2014;168(5):749–756. doi: 10.1016/j.ahj.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Xiao L, Zhang L, Han W, Wang Z, Nattel S. Sex-based transmural differences in cardiac repolarization and ionic-current properties in canine left ventricles. Am J Physiol Heart Circ Physiol. 2006;291(2):H570–580. doi: 10.1152/ajpheart.01288.2005. [DOI] [PubMed] [Google Scholar]
- Yang PC, Clancy CE. Effects of sex hormones on cardiac repolarization. J Cardiovasc Pharmacol. 2010a;56(2):123–129. doi: 10.1097/FJC.0b013e3181d6f7dd. [DOI] [PubMed] [Google Scholar]
- Yang PC, Clancy CE. Gender-based differences in cardiac diseases. J Biomed Res. 2011;25(2):81–89. doi: 10.1016/S1674-8301(11)60010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PC, Clancy CE. In silico Prediction of Sex-Based Differences in Human Susceptibility to Cardiac Ventricular Tachyarrhythmias. Front Physiol. 2012;3:360. doi: 10.3389/fphys.2012.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PC, Kurokawa J, Furukawa T, Clancy CE. Acute effects of sex steroid hormones on susceptibility to cardiac arrhythmias: a simulation study. PLoS Comput Biol. 2010b;6(1):e1000658. doi: 10.1371/journal.pcbi.1000658. [DOI] [PMC free article] [PubMed] [Google Scholar]