Abstract
8-Hydroxyquinoline and derivatives exhibit multifunctional properties, including antioxidant, antineurodegenerative, anticancer, anti-inflammatory and antidiabetic activities. In biological systems, elevation of intracellular calcium can cause calpain activation, leading to cell death. Here, the effect of 8-hydroxyquinoline and derivatives (5-chloro-7-iodo-8-hydroxyquinoline or clioquinol and 8-hydroxy-5-nitroquinoline or nitroxoline) on calpain-dependent (calpain-calpastatin) pathways in human neuroblastoma (SH-SY5Y) cells was investigated. 8-Hydroxyquinoline and derivatives ameliorated high glucose toxicity in SH-SY5Y cells. The investigated compounds, particularly clioquinol, attenuated the increased expression of calpain, even under high-glucose conditions. 8-Hydroxyquinoline and derivatives thus adversely affected the promotion of neuronal cell death by high glucose via the calpain-calpastatin signaling pathways. These findings support the beneficial effects of 8-hydroxyquinolines for further therapeutic development.
Keywords: Calpain, Nitroxoline, 8-Hydroxyquinoline, High glucose, Clioquinol, Neuronal cells
Introduction
Diabetes mellitus (DM) is a complex metabolic disorder featuring chronic hyperglycemia and tremendously impacts human health worldwide. Hyperglycemia contributes to the long-term diabetic complications i.e., retinopathy, nephropathy and neuropathy (Aronsonons, 2008). Epidemiological evidence suggests that patients with DM have a significantly high risk (50–100%) of developing Alzheimer’s disease (Biessels et al., 2006). Diabetic patients exhibit cognitive impairment including damaged verbal memory, diminished mental speed and mental flexibility (Cukierman, Gerstein & Williason, 2005). Chronic hyperglycemia may accelerate the development of Alzheimer’s disease, and many Alzheimer’s patients exhibit impaired fasting glucose (Janson et al., 2004).
Neuronal cells cannot protect themselves from the harmful effects of excess glucose. The most likely mechanism for glucose toxicity is the generation of excess reactive oxygen species (ROS) via multiple mitochondrial and non-mitochondrial pathways (Newsholme et al., 2007). In addition to ROS production, high glucose levels trigger multiple biochemical pathways and toxicity, which contribute to damage to DNA, lipid, proteins and subsequent neurotoxicity (Li et al., 2014). However, the mechanisms underlying the association of high glucose with neurodegeneration remain to be fully elucidated.
Calpain is an intracellular Ca2+-dependent cysteine protease that is activated by increased intracellular Ca2+. Calpain plays a vital role in glucose metabolism, cytoskeletal remodeling for cell cycle regulation and apoptosis, probably as a consequence of a loss of Ca2+ homeostasis (Vosler, Brennan & Chen, 2008). Calpain cleaves and inactivates pro-caspase 9, pro-caspase 3 (Chua, Guo & Li, 2000) and APAF-1 (Lankiewicz et al., 2000). Calpastatin is a specific endogenous calpain inhibitor (Croall & Ersfeld, 2007). Calpain activity underlies the pathophysiology of several neurodegenerative diseases such as ischemia and epilepsy (Vosler, Brennan & Chen, 2008), and overexpression of calpastatin improves ischemia and reperfusion (Maekawa et al., 2003). Interestingly, the increase in calpain expression has been related to Bax, caspase-12, caspase-9 and caspase-3 in dopaminergic neurons (Das, Banik & Ray, 2006; McGinnis et al., 1998). The relative levels of calpain and dopamine in neuron involve the process of neurodegeneration such as Parkinson’s and Alzheimer’s diseases (Chen, Nguyen & Sawmiller, 2011; Carragher, 2006).
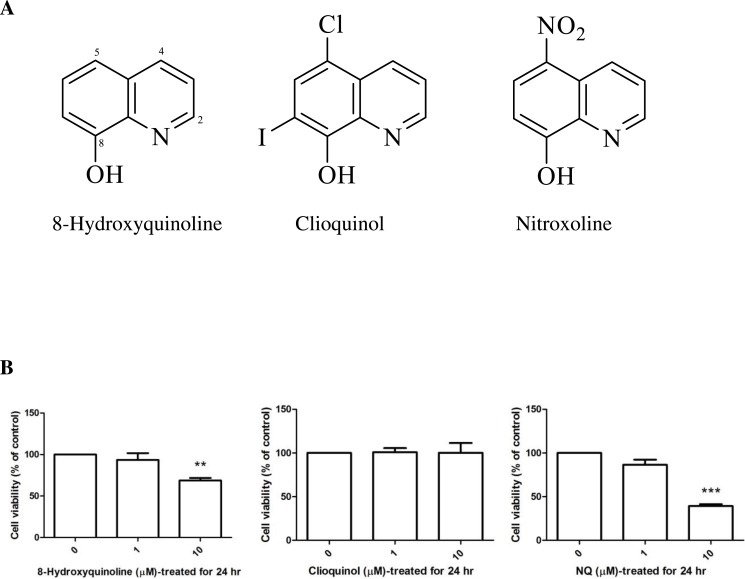
The biometal chelators (Prachayasittikul et al., 2013) 8-hydroxyquinoline, 5-chloro-7-iodo-8-hydroxyquinoline (clioquinol) and 5-nitro-8-hydroxyquinoline (nitroxoline), shown in (Fig. 1A), have been proposed as a potential therapeutic strategy for the treatment of Alzheimer’s disease (Bush, 2008). Clioquinol was identified as a prototype metal-protein-attenuating compound (Barnham, Cheny & Cappai, 2004). The effect of clioquinol is related to its lipophilicity and ability to form relatively stable complexes with zinc (II) and copper (II) ions. Several reports have provided evidence that long-term pretreatment with clioquinol reduces the susceptibility of substantia nigra neurons to neurotoxin (Kaur et al., 2003). These compounds (Fig. 1A) are structurally related and bear 8-hydroxyquinoline as a core structure. Clioquinol is a halogenated derivative, and nitroxoline is the nitro derivative of 8-hydroxyquinoline. 8-Hydroxyquinoline and derivatives are bioavailable antioxidants that can cross the blood–brain barrier and inhibit metal-hydrogen peroxide production (Barnham et al., 2004).
Figure 1. 8-Hydroxyquinoline, clioquinol and nitroxoline.
(A) Chemical structures. (B) Effect of 8-hydroxyquinoline and derivatives on cell viability in SH-SY5Y cells. Cells were treated with 8-hydroxyquinoline and derivatives at 1 and 10 µM for 24 h. Cell viability was measured using the MTT assay and is presented as the percentage of control cells. The results are expressed as the mean ± S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. **P < 0.01 and ***P < 0.001 compared with the control.
Herein, the protective effects of 8-hydroxyquinoline, clioquinol and nitroxoline on human neuroblastoma cells under high glucose were investigated.
Materials and Methods
Chemicals and reagents
Minimum essential medium (MEM), Ham’s F-12 medium, fetal bovine serum (FBS), penicillin and streptomycin were purchased from Gibco BRL (Gaithersburg, MD, USA). Mouse monoclonal anti-actin (catalog number 3700), rabbit polyclonal anti-calpain (catalog number 2539), anti-calpastatin (catalog number 4146) and horseradish peroxidase-conjugated goat anti-mouse IgG and anti-rabbit IgG antibody were supplied by cell signaling (Beverly, MA, USA). Enhanced chemiluminescence (ECL) plus western blotting reagent was purchased from Amersham Biosciences (Piscataway, NJ, USA). The human dopaminergic neuroblastoma (SH-SY5Y) cell line was obtained from American Type Culture Collection (Manassas, VA, USA). SH-SY5Y cells are a thrice cloned subline of bone marrow biopsy-derived line SK-N-SH. SH-SY5Y cell has dopamine-β-hydroxylase activity and express tyrosine hydroxylase. 8-Hydroxyquinoline (99%), clioquinol (≥95%), and nitroxoline (96%) were purchased from Sigma-Aldrich (St Louis, MO, USA).
Cell cultivation
SH-SY5Y cells (passage number less than 25) were cultured in 75-cm2 flasks in MEM-F12 supplemented with 10% heat-inactivated FBS and 100 U/mL penicillin/streptomycin. Cells were maintained at 37 °C in an atmosphere of 5% CO2 and 95% humidified air incubator, and were feed with medium every other day. To perform experiments, cells were seeded in 96-well and 6-well plates and grown to 70–80% confluence. Before the start of treatment, the medium was replaced with MEM-F12 containing 1% (v/v) FBS, as previously described (Dayem et al., 2014; Kovalevich & Langford, 2013). It has been shown that cell incubation with D-glucose for 24 h significantly induced cell apoptosis via the activation of c-Jun N-terminal protein kinase (JNK) and p-38 mitogen-activated protein kinase (MAPK) (Chen et al., 2013; Ho et al., 2000). In case of glucose-treated cells for 2 h, the cells had significantly higher level of ROS accumulation and promoted apoptotic cell death (Wu et al., 2004). Up to date, the mechanism of high glucose contributing to degeneration in neuronal cells remains poorly understood. To investigate the mechanism of high glucose level involved in neuronal cells death, in this study, the cells were treated with D-glucose or D-mannitol at various concentrations (5.5, 30, 60 and 120 mM) for 2 or 24 h, and compared the percentage of cell viability. In some experiments, 8-hydroxyquinoline and derivatives were added to the medium for 2 h prior to an incubation with D-glucose for 24 h. Control untreated cells were incubated with the culture medium. Mannitol was utilized as an osmotic control.
Cell viability assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess neuronal injury after treatment of SH-SY5Y cells with a drug. When MTT is taken up by live cells, it is converted from yellow to dark blue formazan crystals by cellular dehydrogenase (Stockert et al., 2012). MTT in 0.1 mM phosphate buffered saline (PBS) was added to each well and incubated at 37 °C for 4 h. The solution was discarded, and extraction buffer (0.04 N HCl in isopropanol) was added. The optimal densities were measured at a spectral wavelength of 570 nm using a microtiter plate reader.
Western immunoblotting
Treated cells were harvested and lysed by adding lysis buffer and scraped off the plate. Cells were sonicated for 10 s and centrifuged for 15 min at 12,000 g. The supernatants were collected and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein bands were transferred to nitrocellulose membranes and washed with Tris-buffered saline and Tween20 (TBST) for 5 min. The membranes were incubated in a blocking buffer (5% non-fat dry milk in TBST), then washed with TBST and incubated in primary antibodies at 4 °C overnight. After the incubation, the membranes were washed three times with TBST for 5 min and then incubated in HRP-conjugated secondary antibody for 1.5 h, followed by washing three times for 5 min each time with TBST. The blots were developed with ECL Plus Western Blotting detection reagents.
Immunocytochemical analysis
SH-SY5Y cells were seeded on sterile glass coverslips at 37 °C for 24 h and then exposed to D-glucose in the medium containing 1%FBS for 24 h; control cells were incubated with medium for 24 h. The cells were incubated with MitoTracker® Red CMXRos for 30 min. The medium was removed, and the cells were washed with ice-cold PBS. The cells were fixed with 4% paraformaldehyde in PBS for 30 min at 4 °C and washed with PBS three times for 5 min each time. Cells were permeabilized with 1% Triton X-100 in PBS for 10 min at room temperature and rinsed with PBS three times. Non-specific antibody binding sites were blocked by incubating the cells with 10% donkey serum in PBS containing 0.3% Triton X-100 and 1% bovine serum albumin (BSA) for 10 min at room temperature. Cells were incubated with the primary antibody against calpain (1:1,000 in PBS containing 0.3% Triton X-100 and 0.25% BSA) overnight at 4 °C, followed by incubation with fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG (1:200 in PBS containing 0.3% Triton X-100 and 0.25% BSA) for 2 h at room temperature. The cells were washed three times with PBS, and stained slides were mounted using antifade reagent in glycerol buffer (Vector Laboratories, Burlingame, USA) and visualized by fluorescence microscopy (Olympus, Tokyo, Japan).
Statistical analysis
Data are expressed as mean ± S.E.M. Significance was assessed by one-way analysis of variance (ANOVA) followed by a Tukey-Kramer test using SPSS 18 software package for Windows (Chicago, IL, USA). Probability (P) values of less than 0.05 were considered statistically significant.
Results
Effect of high glucose on cell viability of SH-SY5Y cells
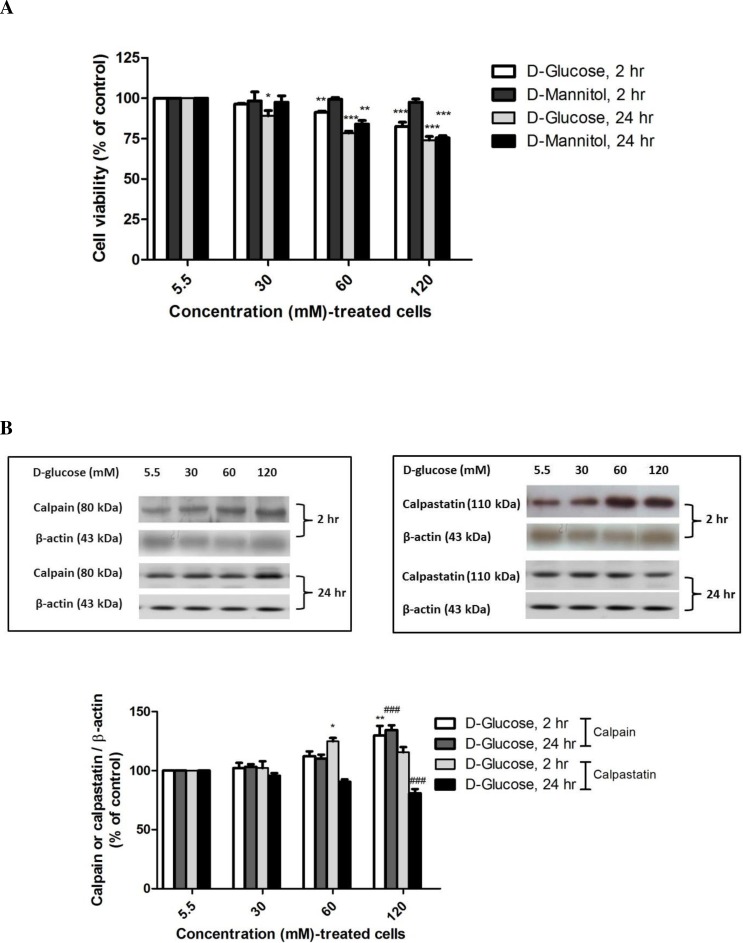
The effect of high-glucose exposure on cell viability was investigated in SH-SY5Y cells using various concentrations of D-glucose and D-mannitol (an osmolality control) medium for 2 h and 24 h. Treatment with D-glucose for 2 h significantly decreased cell viability to 91.31 ± 0.73% at 60 mM (P < 0.01) and to 82.59 ± 2.59% at 120 mM (P < 0.001) compared with normal medium (5.5 mM glucose) (F-value = 30.779) whereas treatment with D-glucose for 24 h significantly decreased cell viability to 89.10 ± 3.23% at 30 mM (P < 0.05), to 78.48 ± 1.16% at 60 mM (P < 0.001), and to 73.97 ± 2.31% at 120 mM (P < 0.001) (F-value = 31.564) (Fig. 2A). To rule out an effect of osmotic stress on SH-SY5Y cells treated with high glucose, cells were incubated with D-mannitol under the same conditions for the indicated time. The differences in cell viability, between cells treated with D-glucose and with D-mannitol at 60 or 120 mM for 24 h were statistically significant. However, high glucose at 60 and 120 mM induced neuronal cell death as a result of hyperglycemia and hyperosmolarity. A decrease in the cell viability of neuronal cells was noted when the cells were treated with high glucose for 2 h. Increasing the ambient D-glucose concentration caused dose- and time-dependent decreases in cell viability. Thus, 120 mM D-glucose was selected to treat neurons in this study because this concentration has been used in many studies of hyperglycemia in vitro (Haslinger et al., 2001; Li, Zhang & Sima, 2003; Song et al., 2015).
Figure 2. High glucose-induced alteration of cell viability, capain and capastatin proteins expression.
Cells treated with D-glucose concentrations (30, 60 and 120 mM) for 2 h and 24 h were compared to cells treated with control medium containing 5.5 mM D-glucose and mannitol as an osmotic control. (A) Cell viability was measured using the MTT assay. (B) The levels of calpain and calpastatin were determined by Western blot analysis. Protein bands were quantified by densitometry, and their differences are represented in the graph as the ratio of calpain and calpastatin to β-actin. The results are expressed as the mean + S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were performed for statistical analysis, *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control at 2 h and ###P < 0.001 compared with the control at 24 h.
Effect of high glucose induced calpain and reduced calpastatin protein levels
To determine if the increase in calcium-dependent pathways induced by high glucose treatment occurs via upregulation of calpain protein, SH-SY5Y cells were incubated with various glucose concentrations (5.5–120 mM) for the indicated time, the cell lysate was collected, and calpain and calpastatin levels were determined by Western blot analysis. Treatment with 120 mM D-glucose for 2 h or 24 h significantly increased calpain levels by 129.69 ± 8.30% (P < 0.01) (F-value = 7.031) and 134.44 ± 3.97% (P < 0.001) (F-value = 31.964) compared with control cells at the same time points (Fig. 2B). These results demonstrate that high glucose induced calpain expression.
Further investigation of calpastatin, a specific endogenous calpain inhibitor, was performed by Western immunoblotting. Interestingly, exposure to 60 mM D-glucose resulted in increased calpastatin expression as early as 2 h (124.80 ± 2.88%, P < 0.01) (F-value = 9.010), whereas 120 mM D-glucose exposure for 24 h significantly decreased calpastatin protein levels to 75.07 ± 4.35% (P < 0.001) (F-value = 11.909) compared with the control (Fig. 2B).
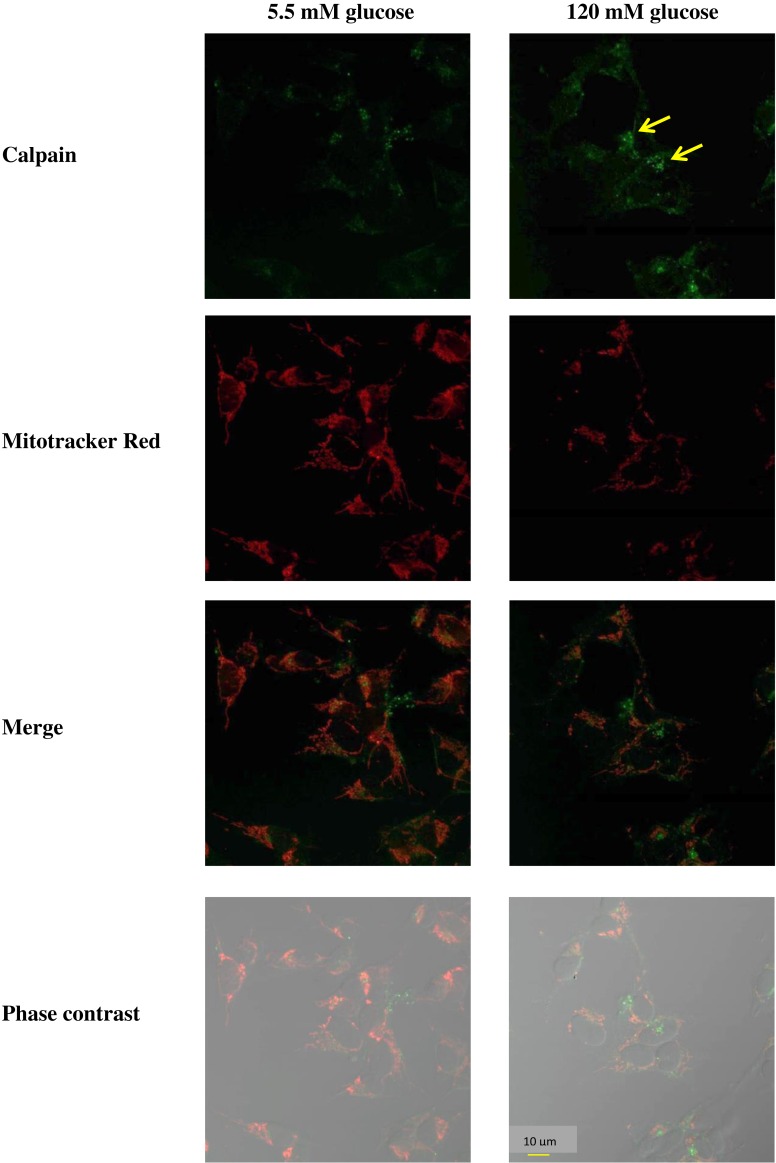
To demonstrate that the observed increase in calpain was related to cell death, an immunofluorescent double-labeling experiment was performed using MitoTracker Red as the mitochondrial marker. Control (5.5 mM D-glucose) cells exhibited weak immunostaining of calpain. However, cells treated with 120 mM D-glucose displayed a bright green speckled appearance that became more intense by 24 h after glucose administration (Fig. 3). Thus, exposure to high glucose resulted in an induction of calpain immunofluorescence staining in SH-SY5Y cells.
Figure 3. Imaging microscopic analysis of SH-SY5Y cells demonstrating the D-glucose-induced increase in calpain expression.
Cells were treated with 120 mM D-glucose for 24 h. The control cells were incubated with the culture medium for 24 h. The green color indicates calpain immunostaining using fluorescein-5-isothiocyanate (FITC)-conjugated anti-IgG.
Cytotoxicity of 8-hydroxyquinoline and derivatives
The cytotoxic effects of 8-hydroxyquinoline and derivatives on cultured cells were assessed at different concentrations using the tetrazolium salt reduction (MTT) assay. No significant cytotoxic effect of 8-hydroxyquinoline and derivatives were evident at 1 µM in SH-SY5Y cells (8-hydroxyquinoline: 93.52 ± 8.15% (F-value = 10.726); clioquinol: 100.8 ± 4.73% (F-value = 0.40); nitroxoline: 86.44 ± 5.87% (F-value = 78.113)) as shown in (Fig. 1B). Cytotoxic effects of 10 µM 8-hydroxyquinoline (68.67 ± 6.37%) and nitroxoline (39.17 ± 2.18%) were observed after treatment for 24 h, and therefore 1 µM was used in subsequent experiments.
Protective effect of 8-hydroxyquinoline and derivatives on high glucose-reduced cell viability
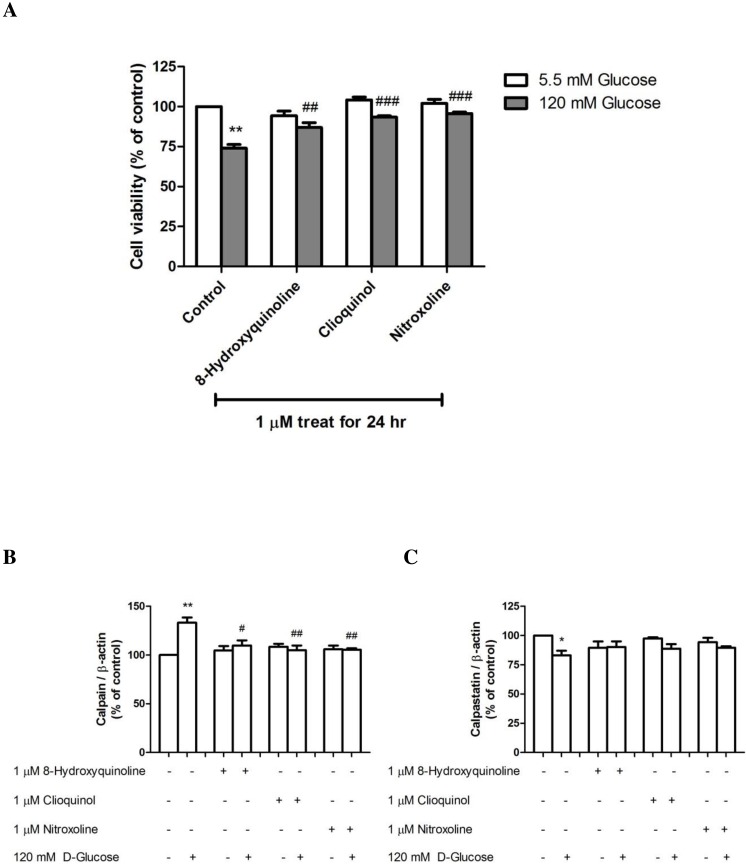
The effects of 8-hydroxyquinoline and derivatives were further investigated by monitoring cell viability changes in response to high-glucose (120 mM) treatment for 24 h. Exposure to 1 µM clioquinol (93.35 ± 0.89%, P < 0.001) or nitroxoline (95.72 ± 0.92%, P < 0.001) significantly increased cell viability compared with high glucose-treated cells (73.97 ± 2.31% P < 0.01) (F-value = 24.262) (Fig. 4A). However, pretreatment with 1 µM 8-hydroxyquinoline also significantly increased cell viability to 86.89 ± 3.06%. The protective effect of the compounds in order of potency was nitroxoline > clioquinol > 8-hydroxyquinoline.
Figure 4. The effect of 8-hydroxyquinoline and derivatives on the high glucose (120 mM) in SH-SY5Y cells.
Cells were treated with high glucose for 24 h. Some cells were pre-treated with 1 µM 8-hydroxyquinoline and derivatives for 2 h prior to incubation with 120 mM high glucose for another 24 h. The control cells were incubated with the culture medium for 24 h. (A) Cell viability was measured using the MTT assay. The results are expressed as the mean ± S.E.M. of four independent experiments. (B) Calpain and (C) calpastatin expressions were determined by Western blot analysis. Protein bands were quantified by densitometry, and the changes are represented in the graph. Calpain and calpastatin expressions are presented as the ratios of calpain or calpastatin/β-actin protein bands. The results are expressed as the mean ± S.E.M. of three independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control and #P < 0.05, ##P < 0.01, ###P < 0.001 compared with high glucose-treated cells.
Effect of 8-hydroxyquinoline and derivatives on high glucose-induced calpain-calpastatin alteration
8-Hydroxyquinoline and derivatives have been reported to exert antidiabetic activity (Prachayasittikul et al., 2013). Calpains are important regulators of the cell cycle and apoptosis, and their activities are dependent on the concentration of calcium in cells. We previously demonstrated that dexamethasone induced neuronal cell death via a calpain-dependent pathway (Suwanjang et al., 2013). In the present study, the effect of 8-hydroxyquinoline and derivatives on high glucose-induced calpain activation was observed (Fig. 4B). Treatment of SH-SY5Y cells with 120 mM D-glucose for 24 h resulted in calpain expression. Pretreatment with 1 µM 8-hydroxyquinoline and derivatives significantly attenuated calpain expression (8-hydroxyquinoline; 109.82 ± 5.28% (P < 0.05) (F-value = 11.489); clioquinol; 104.91 ± 4.95% (P < 0.01) (F-value = 13.919); nitroxoline: 105.47 ± 1.49% (P < 0.01) compared with the high glucose-treated cells (133.19 ± 5.32%, P < 0.001) (F-value = 19.840). A greater protective effect was observed for clioquinol, as evidenced by lower calpain expression under high glucose treatment. However, the protective effect of nitroxoline was comparable to that of clioquinol. By contrast, 8-hydroxyquinoline and derivatives tended to increase the expression of the calpain inhibitor (calpastatin, Fig. 4C) by high glucose (8-hydroxyquinoline: 90.13 ± 4.93% (F-value = 2.840); clioquinol: 88.77 ± 3.88% (F-value = 7.683); nitroxoline: 89.61 ± 1.31% (F-value = 6.570)) compared with the high glucose-treated cells (83.03 ± 4.02%, P < 0.05). Moreover, treatment with 8-hydroxyquinoline and derivatives had no significant effects on the expressions of calpain and calpastatin in untreated control cells.
Discussion
Hyperglycemia is considered a risk factor of neurodegenerative diseases (Kopf & Frolich, 2009). Impairments in signaling mechanisms contribute to increased neuronal cell death. Numerous studies have focused on elucidating the mechanism by which high glucose toxicity enhances death mechanisms. The optimal concentration of glucose for neuronal survival is reportedly in the range of 25–30 mM. Here, cell viability under high-glucose exposure in human neuroblastoma SH-SY5Y cells was investigated. The mechanisms underlying hyperglycemia and hyperosmolarity have been studied extensively. During hyperglycemia, high levels of glucose-induced oxidative stress can cause cellular damage. In addition, excess glucose leads to neurotoxicity via increased apoptosis and inhibition of proliferation. This may activate p38 kinase associated with apoptosis via protein kinase C-dependent and -independent pathways (Igarashi et al., 1999). The results suggest that elevated glucose level initiates harmful mechanisms leading to neuronal cell degeneration (neuropathy). High glucose (120 mM) was reported to affect Ca2+ homeostasis (Kimura, Oike & Ito, 1998). It is also well established that high glucose (120 mM) induced oxidative stress and promoted calcium influx in a variety of cell types including human monocytes (Wuensch et al., 2010) and cardiac cells (Kumar, Kain & Sitasawad, 2012; Ozdemir et al., 2005; Cai et al., 2002).
Impairment of Ca2+ homeostasis is an important factor in the development of neuronal degeneration (Todorovic & Jevtovic-Todorovic, 2014). Under physiological conditions, calpain is localized in the cytosol and is in an inactive form in the absence of calcium. Calpain is activated by cytosolic Ca2+ overload. The dysregulation of intracellular calcium levels is an indicator of neuronal injury through the activation of several enzymes such as calpains and phospholipases as well as mitochondrial alterations (Araujo, Verdasca & Leal, 2004). The calpain system plays a major role in various cellular signaling processes, including signal transduction, cell adhesion and motility, cell growth, differentiation and cell death. Calpain activates both caspase-dependent and caspase-independent pathways to promote apoptosis. In the apoptotic pathway, calpain cleaves apoptotic inducing factor, which activates DNA degradation (Baritaud et al., 2010). Thus, the activation of calpain may have an important role in many diseases such as retinal photoreceptor apoptosis (Mahajan et al., 2012) and ischemia (Rami, 2003). A high concentration of glucose also results in morphological alterations and cell death via processes related to the apoptotic pathway (Allen, Yaqoob & Harwood, 2005). Accumulation of oxidative stress is present in diabetes. Therefore, high glucose can induce cellular hypertrophy by excessive production of ROS. Clinical studies also indicate that high glucose enhances the pathology of diabetes by increasing oxidative stress (Trombetta et al., 2005). In general, ROS are recognized as a main source of molecular damage in hyperglycemia (Piconi et al., 2006).
Calpain activity is inhibited by endogenous calcium-dependent interactions with calpastatin. Calpastatin binds to the active site and inhibits calpain in the presence of calcium (Goll et al., 2003). Specific calpain inhibitors reduce neuronal damage in a number of different systems. The current findings demonstrate that high concentrations of glucose can lead to increased calcium levels and enhance calpain protein expression in a dose- and time-dependent manner. Increased calpain expression has been implicated in vascular inflammation and endothelial leakage in diabetes (Scalia et al., 2007). In addition, calpain plays significant roles in apoptotic processes (Raynaud & Marcilhac, 2006). However, the expression of proteins related to the specific calpain inhibitor (calpastatin) was decreased in SH-SY5Y cells treated with high glucose concentrations. Furthermore, previous studies have suggested that neuronal calpain activity mediates the initiation and expression of methamphetamine- and dexamethasone-induced cell death (Suwanjang et al., 2013; Suwanjang et al., 2012). Several mechanisms have been proposed for the effects of calpain during cell death, including cleavage of pro-caspase 3 and degradation of apoptotic proteins (Camins et al., 2006).
Several studies have revealed a functional association of 8-hydroxyquinoline and derivatives with cancer, inflammation, Alzheimer’s and Parkinson’s diseases (Mao & Schimmer, 2008). Of the tested derivatives, clioquinol is a bioavailable ligand with moderate affinity for copper, zinc and iron. Clioquinol possesses relatively high lipophilicity and crosses the blood–brain barrier. Clioquinol has been observed in brain tissue and cerebrospinal fluid (Bondiolotti et al., 2007) and exhibits neuroprotective effects in MPTP mouse (Kaur et al., 2003) and Alzheimer’s models (Suh, Jensen & Jensen, 2000). Nitroxoline, a nitro derivative of hydroxyquinoline, is used as an antibacterial drug in patients with urinary tract infections (Wagenlehner et al., 2014; Ghoneim, El-Desoky & Abdel-Galeil, 2011). Recently, clioquinol and nitroxoline have been reported to exert anticancer activity against cholangiocarcinoma cells (Chan-On et al., 2015). Clinical trial data suggest that 8-hydroxyquinoline and its derivatives may also have benefits in preventing the development and progression of neurodegeneration. Clioquinol protects against cell death in in vivo and in vitro models of Parkinson’s disease (Wilkins et al., 2009). The induction of calpain in neuronal cells might be closely related to several toxicity mechanisms, including caspase-3 activation (Bastianetto et al., 2011) and oxidative stress (Suwanjang et al., 2013). 8-Hydroxyquinoline and derivatives have been reported as potent antidiabetic agents (Prachayasittikul et al., 2013). The present study demonstrates that treatment of SH-SY5Y cells with 8-hydroxyquinoline and derivatives results in decreased calpain expression and reduced neuronal cell death after high glucose toxicity. Conversely, high-glucose toxicity might be controlled by treatment with 8-hydroxyquinoline and derivatives. Notably, the compounds reduced the expression of calpain in the order clioquinol ≈ nitroxoline > 8-hydroxyquinoline. It has been suggested that 8-hydroxyquinoline and derivatives, especially clioquinol prevent Ca2+ influx and calcium signal in Alzheimer’s (LeVine et al., 2009; Abramov, Canevari & Dunchen, 2005; Lin, Bhatia & Lal, 2003; Cherny et al., 2001) and Parkinson’s diseases (Cacciatore et al., 2013). Furthermore, it is also reasonable that 8-hydroxyquinoline and derivatives decrease calpain but increase calpastatin expressions via their antioxidant activities and reduce intracellular calcium level in high glucose toxicity. In addition, the decrease in calcium level may be enhanced by metal chelating property of 8-hydroxyquinoline and derivatives (Prachayasittikul et al., 2013).
Conclusions
This study reveals that 8-hydroxyquinoline and derivatives offer partial neuroprotection against high glucose toxicity and modulate the balance between calpain and calpastatin expressions. These findings provide a foundation for the further therapeutic development of 8-hydroxyquinoline compounds.
Supplemental Information
B) Cells were treated with 8-hydroxyquinoline and derivative at 1 and 10 µM for 24 h. Cell viability was measured using the MTT assay and is presented as the percentage of control cells. The results are expressed as the mean ± S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. **P < 0.01 and ***P < 0.001 compared with control.
Cells treated with D-glucose concentrations (30, 60 and 120 mM) for 2 h and 24 h were compared to cells treated with control medium containing 5.5 mM D-glucose and mannitol as an osmotic control. Cell viability was measured using the MTT assay. The results are expressed as the mean + S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were performed for statistical analysis, *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control at 2 h and ###P < 0.001 compared with control at 24 h.
Cells treated with D-glucose concentrations (30, 60 and 120 mM) for 2 h and 24 h were compared to cells treated with control medium containing 5.5 mM D-glucose and mannitol as an osmotic control. The results are expressed as the mean + S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were performed for statistical analysis, *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control at 2 h and ###P < 0.001 compared with control at 24 h.
D-glucose concentrations (30, 60 and 120 mM) for 2 h and 24 h were compared to cells treated with control medium containing 5.5 mM D-glucose. The levels of calpain was determined by Western blot analysis. Protein bands were quantified by densitometry, and their differences are represented in the graph as the ratio of calpain to β-actin. The results are expressed as the mean + S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were performed for statistical analysis, *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control at 2 h and ###P < 0.001 compared with control at 24 h.
Cells treated with D-glucose concentrations (30, 60 and 120 mM) for 2 h and 24 h were compared to cells treated with control medium containing 5.5 mM D-glucose. The levels of calpastatin was determined by Western blot analysis. Protein bands were quantified by densitometry, and their differences are represented in the graph as the ratio of calpastatin to β-actin. The results are expressed as the mean + S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were performed for statistical analysis, *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control at 2 h and ###P < 0.001 compared with control at 24 h.
Cells were treated with high glucose for 24 h. Some cells were pre-treated with 1 µM 8-hydroxyquinoline and derivatives for 2 h prior to incubation with 120 mM high glucose for another 24 h. The control cells were incubated with culture medium for 24 h. A, Cell viability was measured using the MTT assay. The results are expressed as the mean ± S.E.M. of four independent experiments. The results are expressed as the mean ± S.E.M. of three independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control and #P < 0.05, ##P < 0.01, ###P < 0.001 compared with high glucose-treated cells.
Cells were treated with high glucose for 24 h. Some cells were pre-treated with 1 µM 8-hydroxyquinoline for 2 h prior to incubation with 120 mM high glucose for another 24 h. The control cells were incubated with culture medium for 24 h. Calpain and calpastatin expressions were determined by Western blot analysis. Protein bands were quantified by densitometry, and the changes are represented in the graph. Calpain and calpastatin expression are presented as the ratios of calpain or calpastatin/β-actin protein bands. The results are expressed as the mean ± S.E.M. of three independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control and #P < 0.05, ##P < 0.01, ###P < 0.001 compared with high glucose-treated cells.
Cells were treated with high glucose for 24 h. Some cells were pre-treated with 1 µM clioquinol for 2 h prior to incubation with 120 mM high glucose for another 24 h. The control cells were incubated with culture medium for 24 h. Calpain and calpastatin expressions were determined by Western blot analysis. Protein bands were quantified by densitometry, and the changes are represented in the graph. Calpain and calpastatin expression are presented as the ratios of calpain or calpastatin/β-actin protein bands. The results are expressed as the mean ± S.E.M. of three independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control and #P < 0.05, ##P < 0.01, ###P < 0.001 compared with high glucose-treated cells.
Cells were treated with high glucose for 24 h. Some cells were pre-treated with 1 µM nitroxoline for 2 h prior to incubation with 120 mM high glucose for another 24 h. The control cells were incubated with culture medium for 24 h. Calpain and calpastatin expressions were determined by Western blot analysis. Protein bands were quantified by densitometry, and the changes are represented in the graph. Calpain and calpastatin expression are presented as the ratios of calpain or calpastatin/β-actin protein bands. The results are expressed as the mean ± S.E.M. of three independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control and #P < 0.05, ##P < 0.01, ###P < 0.001 compared with high glucose-treated cells.
Acknowledgments
We thank Ms.Chayanit Sirisuwat for collecting the samples.
Funding Statement
This project was supported by Mahidol University, Thailand, to Wilasinee Suwanjang, and by the Office of the Higher Education Commission, Mahidol University, under the National Research University Initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Wilasinee Suwanjang conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables.
Supaluk Prachayasittikul and Virapong Prachayasittikul conceived and designed the experiments, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as Supplementary Files.
References
- Abramov, Canevari & Dunchen (2005).Abramov AY, Canevari L, Dunchen MR. Amyloid β peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. Journal of Neuroscience. 2005;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, Yaqoob & Harwood (2005).Allen D, Yaqoob M, Harwood S. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. Journal of Nutritional Biochemistry. 2005;16:705–713. doi: 10.1016/j.jnutbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Araujo, Verdasca & Leal (2004).Araujo I, Verdasca M, Leal E. Early calpain-mediated proteolysis following AMPA receptor activation compromises neuronal survival in cultured hippocampal neurons. Journal of Neurochemistry. 2004;91:1322–1331. doi: 10.1111/j.1471-4159.2004.02811.x. [DOI] [PubMed] [Google Scholar]
- Aronsonons (2008).Aronsonons D. Hyperglycemia and the pathobiology of diabetic complication. Advances in Cardiology. 2008;45:1–16. doi: 10.1159/000115118. [DOI] [PubMed] [Google Scholar]
- Baritaud et al. (2010).Baritaud M, Boujrad H, Lorenzo HK, Krantic S, Susin Sa. Histone H2AX: the missing link in AIF-mediated caspase-independent programmed necrosis. Cell Cycle. 2010;9:3166–3173. doi: 10.4161/cc.9.16.12887. [DOI] [PubMed] [Google Scholar]
- Barnham, Cheny & Cappai (2004).Barnham K, Cheny R, Cappai R. Metal protein attenuating compounds (MPACs) for the treatment of Alzheimer’s disease. Drug Design Reviews Online. 2004;1:75–82. doi: 10.2174/1567269043480690. [DOI] [Google Scholar]
- Barnham et al. (2004).Barnham K, Haeffner F, Ciccotosto G, Curtain C, Tew D, Mavros C, Beyreuther K, Carrington D, Cherny R, Cappai R, Bush A. Tyrosine gated electron transfer is key to the toxic mechanism of Alzheimer’s disease beta-amyloid. FASEB Journal. 2004;18:1427–1429. doi: 10.1096/fj.04-1890fje. [DOI] [PubMed] [Google Scholar]
- Bastianetto et al. (2011).Bastianetto S, Krantic S, Chabot J, Quirion R. Possible involvement of programmed cell death pathways in the neuro-protective action of polyphenols. Current Alzheimer Research. 2011;8:445–451. doi: 10.2174/156720511796391854. [DOI] [PubMed] [Google Scholar]
- Biessels et al. (2006).Biessels G, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabeties mellitus: a systematic review. The Lancet Neurology. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Bondiolotti et al. (2007).Bondiolotti G, Sala M, Pollera C, Gervasoni M, Puricelli M, Ponti W, Bareggi S. Pharmacokinetics and distribution of clioquinol in golgen hamsters. Journal of Pharmacy and Pharmacology. 2007;59:387–393. doi: 10.1211/jpp.59.3.0008. [DOI] [PubMed] [Google Scholar]
- Bush (2008).Bush A. Drug development based on the metals hypothesis of Alzheimer’s disease. Journal of Alzheimer’s Disease. 2008;15:223–240. doi: 10.3233/jad-2008-15208. [DOI] [PubMed] [Google Scholar]
- Cacciatore et al. (2013).Cacciatore I, Fornasari E, Baldassarre L, Cornacchia C, Fulle S, Filippo ESD, Pietrangelo T, Pinnen F. A potent (R)-alpha-bis-lipoyl derivative containing 8-hydroxyquinoline scaffold: synthesis and biological evaluation of its neuroprotective capabilities in SH-SY5Y human neuroblastoma cells. Pharmaceuticals. 2013;6:54–69. doi: 10.3390/ph6010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai et al. (2002).Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome c-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- Camins et al. (2006).Camins A, Verdaguer E, Folch J, Pallas M. Involvement of calpain activation in neurodegenerative processes. CNS Drug Reviews. 2006;12:135–148. doi: 10.1111/j.1527-3458.2006.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher (2006).Carragher NO. Calpain inhibition: a therapeutic strategy _targeting multiple disease states. Current Pharmaceurical Design. 2006;12:615–638. doi: 10.2174/138161206775474314. [DOI] [PubMed] [Google Scholar]
- Chan-On et al. (2015).Chan-On W, Huyen NP, Songtawee N, Suwanjang W, Prachayasittikul S, Prachayasittikul V. Quinoline-based clioquinol and nitroxoline exhibit anticancer activity inducing FoxM1 inhibition in cholangiocarcinoma cells. Journal of Drug Design, Development and Therapy. 2015;9:2033–2047. doi: 10.2147/DDDT.S79313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2013).Chen J, Guo Y, Chen W, Chen R, Liu T, Chen Z, Tan S. High glucose induces apoptosis and suppresses proliferation of adult rat neural stem cells following in vitro ischemia. BMC Neuroscience. 2013;14:24. doi: 10.1186/1471-2202-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Nguyen & Sawmiller (2011).Chen M, Nguyen HT, Sawmiller DR. What to look for beyond pathogenic factors in senile dementia? A functional deficiency of Ca2+ signaling. Journal of Alzheimer’s Disease. 2011;27:679–689. doi: 10.3233/JAD-2011-111142. [DOI] [PubMed] [Google Scholar]
- Cherny et al. (2001).Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/S0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Chua, Guo & Li (2000).Chua B, Guo K, Li P. Direct cleavage by the calcium activated protease calpain can lead to inactivation of caspases. Journal of Biological Chemistry. 2000;275:5131–5135. doi: 10.1074/jbc.275.7.5131. [DOI] [PubMed] [Google Scholar]
- Croall & Ersfeld (2007).Croall D, Ersfeld K. The calpains: modular designs and functional diversity. Genome Biology. 2007;8:218. doi: 10.1186/gb-2007-8-6-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman, Gerstein & Williason (2005).Cukierman T, Gerstein H, Williason J. Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabeteologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- Das, Banik & Ray (2006).Das A, Banik NL, Ray SK. Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. International Journal of Cancer. 2006;119:2575–2585. doi: 10.1002/ijc.22228. [DOI] [PubMed] [Google Scholar]
- Dayem et al. (2014).Dayem AA, Kim BW, Gurunathan S, Choi HY, Yang G, Saha SK, Han D, Han J, Kim K, Kim JH, Cho SG. Biologically synthesized silver nanoparticles induce neuronal differentiation of SH-SY5Y cells via modulation of reactive oxygen species, phosphatases, and kinase signaling pathways. Journal of Biotechnology. 2014;9:934–943. doi: 10.1002/biot.201400555. [DOI] [PubMed] [Google Scholar]
- Ghoneim, El-Desoky & Abdel-Galeil (2011).Ghoneim MM, El-Desoky HS, Abdel-Galeil MM. Electrochemistry of the antibacterial and antifungal drug nitroxoline and its determination in bulk form, pharmaceutical formulation and human blood. Bioelectrochemistry. 2011;80:162–168. doi: 10.1016/j.bioelechem.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Goll et al. (2003).Goll DE, Thompson VF, Li H, Wei WEL, Cong J. The calpain system. Physiological Reviews. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Haslinger et al. (2001).Haslinger B, Mandl-Weber S, Sellmayer A, Lederer S, Sitter T. Effect of high glucose concentration on the synthesis of monocyte chemoattractant protein-1 in human peritoneal mesothelial cells: involvement of protein kinase C. Nephron. 2001;87:346–351. doi: 10.1159/000045941. [DOI] [PubMed] [Google Scholar]
- Ho et al. (2000).Ho FM, Liu SH, Liau CS, Huang PJ, Lin-Shiau SY. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH2-terminal kinase and caspase-3. Circulation. 2000;101:2618–2624. doi: 10.1161/01.CIR.101.22.2618. [DOI] [PubMed] [Google Scholar]
- Igarashi et al. (1999).Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang Z, Yamauchi T, Kuboki K, Meier M, Rhodes C, King G. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. Journal of Clinical Investigation. 1999;103:185–195. doi: 10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson et al. (2004).Janson J, Laedtke T, Parisi J, O’Brien P, Peterson R. Increased risk of type 2 diabetes in Alzheimer’s disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- Kaur et al. (2003).Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo J, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal M, Dimonte D, Volitaskis I, Ellerby L, Cherny R, Bush A, Anderson J. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron. 2003;37:899–909. doi: 10.1016/S0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Kimura, Oike & Ito (1998).Kimura C, Oike M, Ito Y. Acute glucose overload abolishes Ca2+ oscillation in cultured endothelial cells from bovine aorta: a possible role of superoxide anion. Circulation Research. 1998;82:677–685. doi: 10.1161/01.RES.82.6.677. [DOI] [PubMed] [Google Scholar]
- Kopf & Frolich (2009).Kopf D, Frolich L. Risk of incident Alzheimer’s disease in diabetic patients: a systemic review of prospective trials. Journal of Alzheimer’s Disease. 2009;16:677–685. doi: 10.3233/JAD-2009-1011. [DOI] [PubMed] [Google Scholar]
- Kovalevich & Langford (2013).Kovalevich J, Langford D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods in Molecular Biology. 2013;1078:9–21. doi: 10.1007/978-1-62703-640-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, Kain & Sitasawad (2012).Kumar S, Kain V, Sitasawad SL. High glucose-induced Ca2+ overload and oxidative stress contribute to apoptosis of cardiac cells through mitochondrial dependent and independent pathways. Biochimica et Biophysica Acta. 2012;1820:907–920. doi: 10.1016/j.bbagen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Lankiewicz et al. (2000).Lankiewicz S, Luerjens CM, Bui NT, Krohn A, Poppe M, Cole G, Saido T, Prehn J. Activation of calpain I converts excitotoxic neuronal death into a caspase-independent cell death. Journal of Biological Chemistry. 2000;275:17064–17071. doi: 10.1074/jbc.275.22.17064. [DOI] [PubMed] [Google Scholar]
- LeVine et al. (2009).LeVine H, Ding Q, Walker JA, Voss RS, Augelli-Szafran CE. Clioquinol and other hydroxyquinoline derivatives inhibit Aβ (1-42) oligomer assembly. Neuroscience Letters. 2009;465:99–103. doi: 10.1016/j.neulet.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2014).Li Y, Xu S, Zhang Q, Li L, Lai L, Zheng T, Su J, Yang N, Li Y. Cytotoxicity study on SH-SY5Y cells cultured at high glucose levels and treated with bupivacaine. Molecular Medicine Reports. 2014;9:515–520. doi: 10.3892/mmr.2013.1843. [DOI] [PubMed] [Google Scholar]
- Li, Zhang & Sima (2003).Li Z, Zhang W, Sima A. C-peptide enhances insulin-mediated cell growth and protection against high glucose–induced apoptosis in SH-SY5Y cells. Diabetes/Metabolism Research and Reviews. 2003;19:375–385. doi: 10.1002/dmrr.389. [DOI] [PubMed] [Google Scholar]
- Lin, Bhatia & Lal (2003).Lin H, Bhatia R, Lal R. Amyloid β protein forms ion channels: implications for Alzheimer’s disease pathophysiology. FASEB Journal. 2003;15:2433–2444. doi: 10.1096/fj.01-0377com. [DOI] [PubMed] [Google Scholar]
- Maekawa et al. (2003).Maekawa A, Lee J, Nagaya T, Kamiya K, Yasui K, Horiba M. Overexpression of calpastatin by gene transfer prevents troponin I degradation and ameliorates contractile dysfunction in rat hearts subjected to ischemia/reperfusion. Journal of Molecular and Cellular Cardiology. 2003;35:1277–1284. doi: 10.1016/S0022-2828(03)00238-4. [DOI] [PubMed] [Google Scholar]
- Mahajan et al. (2012).Mahajan V, Skeie J, Bassuk A, Fingert J, Braun T, Daggett H, Folk J, Sheffield V, Stone E. Calpain-5 mutations cause autoimmune uveitis, retinal neovascularization, and photoreceptor degeneration. PLoS Genetics. 2012;10:e2389. doi: 10.1371/journal.pgen.1003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao & Schimmer (2008).Mao X, Schimmer A. The toxicology of clioquinol. Toxicology Letters. 2008;182:1–6. doi: 10.1016/j.toxlet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- McGinnis et al. (1998).McGinnis KM, Whitton MM, Gnegy ME, Wang KKW. Calcium/Calmodulin-dependent protein kinase IVis cleaved by caspase-3 and calpain in SH-SY5Y human neuroblastoma cells undergoing apoptosis. Journal of Biological Chemistry. 1998;273:19993–20000. doi: 10.1074/jbc.273.32.19993. [DOI] [PubMed] [Google Scholar]
- Newsholme et al. (2007).Newsholme P, Haber E, Hirabara S, Rebelato E, Procopio J, Morgan D, Oliveira-Emilio H, Carpinelli A, Curi R. Diabetes associated cell stress and dysfunction: role of mitodrial and non-mitochondrial ROS production and activity. Journal of Physiology. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir et al. (2005).Ozdemir S, Ugur M, Gurdal H, Turan B. Treatment with AT(1) receptor blocker restores diabetes-induced alterations in intracellular Ca2+ transients and contractile function of rat myocardium. Archives Biochemistry and Biophysics. 2005;435:166–174. doi: 10.1016/j.abb.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Piconi et al. (2006).Piconi L, Qualiaro L, Assaloni R, DA R, Maier A, Zuodar G, Ceriello A. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes/Metabolism Research and Reviews. 2006;22:198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- Prachayasittikul et al. (2013).Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications. Drug Design, Development and Therapy. 2013;7:1157–1178. doi: 10.2147/DDDT.S49763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami (2003).Rami A. Ischemic neuronal death in the rat hippocampus: the calpain-calpastatin-caspase hypothesis. Neurobiology of Disease. 2003;13:75–88. doi: 10.1016/S0969-9961(03)00018-4. [DOI] [PubMed] [Google Scholar]
- Raynaud & Marcilhac (2006).Raynaud F, Marcilhac A. Implication of calpain in neuronal apoptosis. A possible regulation of Alzheimer’s disease. FEBS Journal. 2006;273:3437–3443. doi: 10.1111/j.1742-4658.2006.05352.x. [DOI] [PubMed] [Google Scholar]
- Scalia et al. (2007).Scalia R, Gong Y, Berzins B, Zhao L, Sharma K. Hyperglycemia is a major determinant of albumin permeability in diabetic microcirculation: the role of mu-calpain. Diabetes. 2007;56:1842–1849. doi: 10.2337/db06-1198. [DOI] [PubMed] [Google Scholar]
- Song et al. (2015).Song J, Kang S, Kim E, Kim C-H, Song H-T, Lee J. Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: an in vivo and in vitro study. Cell Death and Disease. 2015;6:e2389. doi: 10.1038/cddis.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert et al. (2012).Stockert JC, Blazques-Castro A, Canete M, Horobin RW, Villanueva A. MTT assay for cell viability: intracellular localization of the formazan product is in lipid droplets. Acta Histochemica. 2012;114:785–796. doi: 10.1016/j.acthis.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Suh, Jensen & Jensen (2000).Suh S, Jensen K, Jensen M. Histochemically reactive zinc in amyloid plaques, angiopathy and degenerating neurons of Alzheimer’s diseased brains. Brain Research. 2000;852:274–278. doi: 10.1016/S0006-8993(99)02096-X. [DOI] [PubMed] [Google Scholar]
- Suwanjang et al. (2013).Suwanjang W, Abramov A, Govitrapong P, Chetsawang B. Melatonin attenuates dexamethasone toxicity-induced oxidative stress, calpain and caspase activation in human neuroblastoma SH-SY5Y cells. Journal of Steroid Biochemistry and Molecular Biology. 2013;138:116–122. doi: 10.1016/j.jsbmb.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Suwanjang et al. (2012).Suwanjang W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B. Calpastatin reduces calpain and caspase activation in methaphetamine-induced toxicity in human neuroblastoma SH-SY5Y cultured cells. Neuroscience Letters. 2012;526:49–53. doi: 10.1016/j.neulet.2012.07.066. [DOI] [PubMed] [Google Scholar]
- Todorovic & Jevtovic-Todorovic (2014).Todorovic S, Jevtovic-Todorovic V. _targeting of CaV3.2 T-type calcium channels in peripheral sensory neurons for the treatment of painful diabetic neuropathy. Pflugers Archiv. 2014;466:701–706. doi: 10.1007/s00424-014-1452-z. [DOI] [PubMed] [Google Scholar]
- Trombetta et al. (2005).Trombetta M, Spiazzi G, Zoppini G, Muggeo M. Review article: type 2 diabetes and chronic liver disease in the Verona diabetes study. Alimentary Pharmacology and Therapeutics. 2005;2:24–27. doi: 10.1111/j.1365-2036.2005.02590.x. [DOI] [PubMed] [Google Scholar]
- Vosler, Brennan & Chen (2008).Vosler P, Brennan C, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Molecular Neurobiology. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenlehner et al. (2014).Wagenlehner FME, Münch F, Pilatz A, Bärmann B, Weidner W, Wagenlehner CM, Straubinger M, Blenk H, Pfister W, Kresken M, Naber KG. Urinary concentration and antibacterial activities of nitroxline at 250 milligrams versus trimethoprim at 200 milligrams against uropathogens in healthy volunteers. Antimicrobial Agents and Chemotherapy. 2014;58:713–721. doi: 10.1128/AAC.02147-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins et al. (2009).Wilkins S, Masters C, Bush A, Cherny R, Finkelstein D. Clioquinol protects against cell death in Parkinson’s disease models in vivo and in vitro. Advances in Behavioral Biology. 2009;58:431–442. doi: 10.1007/978-1-4419-0340-2_33. [DOI] [Google Scholar]
- Wu et al. (2004).Wu L, Nicholson W, Knobel SM, Steffner RJ, May JM, Piston DW, Powers AC. Oxidative stress is a mediator of glucose toxicity in insulin-secreting pancreatic islet cell lines. Journal of Biological Chemistry. 2004;279:12126–12134. doi: 10.1074/jbc.M307097200. [DOI] [PubMed] [Google Scholar]
- Wuensch et al. (2010).Wuensch T, Thilo F, Krueger K, Scholze A, Ristow M, Tepel M. High glucose-induced oxidative stress increases transient receptor potential channel expression in human monocytes. Diabetes. 2010;59:844–849. doi: 10.2337/db09-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
B) Cells were treated with 8-hydroxyquinoline and derivative at 1 and 10 µM for 24 h. Cell viability was measured using the MTT assay and is presented as the percentage of control cells. The results are expressed as the mean ± S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. **P < 0.01 and ***P < 0.001 compared with control.
Cells treated with D-glucose concentrations (30, 60 and 120 mM) for 2 h and 24 h were compared to cells treated with control medium containing 5.5 mM D-glucose and mannitol as an osmotic control. Cell viability was measured using the MTT assay. The results are expressed as the mean + S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were performed for statistical analysis, *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control at 2 h and ###P < 0.001 compared with control at 24 h.
Cells treated with D-glucose concentrations (30, 60 and 120 mM) for 2 h and 24 h were compared to cells treated with control medium containing 5.5 mM D-glucose and mannitol as an osmotic control. The results are expressed as the mean + S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were performed for statistical analysis, *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control at 2 h and ###P < 0.001 compared with control at 24 h.
D-glucose concentrations (30, 60 and 120 mM) for 2 h and 24 h were compared to cells treated with control medium containing 5.5 mM D-glucose. The levels of calpain was determined by Western blot analysis. Protein bands were quantified by densitometry, and their differences are represented in the graph as the ratio of calpain to β-actin. The results are expressed as the mean + S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were performed for statistical analysis, *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control at 2 h and ###P < 0.001 compared with control at 24 h.
Cells treated with D-glucose concentrations (30, 60 and 120 mM) for 2 h and 24 h were compared to cells treated with control medium containing 5.5 mM D-glucose. The levels of calpastatin was determined by Western blot analysis. Protein bands were quantified by densitometry, and their differences are represented in the graph as the ratio of calpastatin to β-actin. The results are expressed as the mean + S.E.M. of four independent experiments. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were performed for statistical analysis, *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control at 2 h and ###P < 0.001 compared with control at 24 h.
Cells were treated with high glucose for 24 h. Some cells were pre-treated with 1 µM 8-hydroxyquinoline and derivatives for 2 h prior to incubation with 120 mM high glucose for another 24 h. The control cells were incubated with culture medium for 24 h. A, Cell viability was measured using the MTT assay. The results are expressed as the mean ± S.E.M. of four independent experiments. The results are expressed as the mean ± S.E.M. of three independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control and #P < 0.05, ##P < 0.01, ###P < 0.001 compared with high glucose-treated cells.
Cells were treated with high glucose for 24 h. Some cells were pre-treated with 1 µM 8-hydroxyquinoline for 2 h prior to incubation with 120 mM high glucose for another 24 h. The control cells were incubated with culture medium for 24 h. Calpain and calpastatin expressions were determined by Western blot analysis. Protein bands were quantified by densitometry, and the changes are represented in the graph. Calpain and calpastatin expression are presented as the ratios of calpain or calpastatin/β-actin protein bands. The results are expressed as the mean ± S.E.M. of three independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control and #P < 0.05, ##P < 0.01, ###P < 0.001 compared with high glucose-treated cells.
Cells were treated with high glucose for 24 h. Some cells were pre-treated with 1 µM clioquinol for 2 h prior to incubation with 120 mM high glucose for another 24 h. The control cells were incubated with culture medium for 24 h. Calpain and calpastatin expressions were determined by Western blot analysis. Protein bands were quantified by densitometry, and the changes are represented in the graph. Calpain and calpastatin expression are presented as the ratios of calpain or calpastatin/β-actin protein bands. The results are expressed as the mean ± S.E.M. of three independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control and #P < 0.05, ##P < 0.01, ###P < 0.001 compared with high glucose-treated cells.
Cells were treated with high glucose for 24 h. Some cells were pre-treated with 1 µM nitroxoline for 2 h prior to incubation with 120 mM high glucose for another 24 h. The control cells were incubated with culture medium for 24 h. Calpain and calpastatin expressions were determined by Western blot analysis. Protein bands were quantified by densitometry, and the changes are represented in the graph. Calpain and calpastatin expression are presented as the ratios of calpain or calpastatin/β-actin protein bands. The results are expressed as the mean ± S.E.M. of three independent experiments. One-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test were performed for statistical analysis. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the control and #P < 0.05, ##P < 0.01, ###P < 0.001 compared with high glucose-treated cells.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as Supplementary Files.