ABSTRACT
Caloric starvation, as well as various diets, has been proposed to increase the oxidative DNA damage induced by radiotherapy (RT). However, some diets could have dual effects, sometimes promoting cancer growth, whereas proposing caloric restriction may appear counterproductive during RT considering that the maintenance of weight is a major factor for the success of this therapy. A systematic review was performed via a PubMed search on RT and fasting, or caloric restriction, ketogenic diet (>75% of fat-derived energy intake), protein starvation, amino acid restriction, as well as the Warburg effect. Twenty-six eligible original articles (17 preclinical studies and 9 clinical noncontrolled studies on low-carbohydrate, high-fat diets popularized as ketogenic diets, representing a total of 77 patients) were included. Preclinical experiments suggest that a short period of fasting prior to radiation, and/or transient caloric restriction during treatment course, can increase tumor responsiveness. These regimens promote accumulation of oxidative lesions and insufficient repair, subsequently leading to cancer cell death. Due to their more flexible metabolism, healthy cells should be less sensitive, shifting their metabolism to support survival and repair. Interestingly, these regimens might stimulate an acute anticancer immune response, and may be of particular interest in tumors with high glucose uptake on positron emission tomography scan, a phenotype associated with poor survival and resistance to RT. Preclinical studies with ketogenic diets yielded more conflicting results, perhaps because cancer cells can sometimes metabolize fatty acids and/or ketone bodies. Randomized trials are awaited to specify the role of each strategy according to the clinical setting, although more stringent definitions of proposed diet, nutritional status, and consensual criteria for tumor response assessment are needed. In conclusion, dietary interventions during RT could be a simple and medically economical and inexpensive method that may deserve to be tested to improve efficiency of radiation.
Keywords: radiotherapy, fasting, caloric restriction, ketogenic diet, Warburg effect, immunity
Dietary interventions may increase the oxidative DNA damage induced by radiotherapy: summary of published preclinical and clinical studies.
Introduction
Background
The effect of conventional radiotherapy (RT) is mostly due to oxidative damage induced by reactive oxygen species (ROS) released during water radiolysis, in particular hydrogen peroxide (1). Oxygen is the best radiosensitizer, which favors ROS formation and oxidative stress–mediated damage (2). However, cancer tissues are often hypoxic (3). Therefore, to increase oxygen concentration in tumors, several pro-oxidative strategies have been proposed, such as hyperbaric oxygen therapy, blood transfusions, erythropoietin injection (4, 5), as well as hypoxia-inducible factor 1 (HIF-1) inhibitors (6) and oxygen mimetics compounds (7, 8). In recent times, there has been increasing interest by the general public in the use of natural products and diet regimens for cancer prevention and improvement in the efficacy and tolerability of cancer treatment.
However, the impact of dietary measures on response to radiation has been poorly elucidated. It has been reported that nutrient deprivation stimulates hydrogen peroxide production in cancer cells and promotes oxidative stress in response to RT (9, 10). However, since maintenance of an appropriate BMI is a major determinant of RT efficacy (11–13), it may appear counterproductive to propose dietary restrictions during this period. In a recent review of >200 cancer patients consuming ketogenic diets (KDs) (14), it was concluded that the “probability of achieving an anti-tumor effect seemed greater with KDs than that of causing serious side effects.” To clarify these issues, we conducted a systematic review examining the potential benefit of various nutritional interventions during RT, such as short fasting (SF), caloric restriction (CR), KD, and protein/amino acid restriction, to increase tumor response and survival.
Hallmarks of tumor cell metabolism
Cancer cells, even in presence of normal oxygen concentration, consume great amounts of glucose and release lactic acid, a phenomenon called the “Warburg effect” (15, 16). This metabolic anomaly is sustained by a complex interaction among different factors such as inactivation of onco-suppressor genes (p53), activations of oncogenic (MYC and RAS) and hypoxia-related (HIF-1) pathways, promotion of the proliferative signaling axis mediated by the phosphatidylinositol 3-kinase/protein kinase B/mammalian _target of rapamycin (PI3K/AKT/mTOR), as well as loss of mitochondria, dysfunction of the tricarboxylic acid cycle (TCA) cycle, and of the respiratory chain (17, 18). The large consumption of glucose and calories sustains biosynthesis while mitochondrial ATP, citrate and ROS production are maintained in adequate ranges for active proliferation (19). Lactate, released in the microenvironment, promotes invasiveness, suppresses immune response, and can fuel the oxidative metabolism of well-oxygenated cancer cells. This recycling pathway allows sparing of glucose for most hypoxic cancer cells (20). The presence of these metabolic features is in accordance with the observed correlation between a high uptake of 18F-fluorodeoxyglucose (18FDG) by tumors in positron emission tomography (18FDG-PET) scan and a highly glycolytic metabolism, translating into increased resistance to chemotherapy (CT) and RT (21) and poor survival (22, 23).
Definition of dietary interventions
SF is a complete cessation of all caloric intake for a limited interval of time (24), while CR is usually defined as a reduction in calorie intake of ≥20–30%, without restriction of water (25). CR is usually limited to a maximum of 3 d; however, repeated cycles are possible (26, 27). Severe and prolonged CR (<600–800 kcal/d) corresponds to a very low caloric intake. For instance, a low-caloric diet provides between 10 and 20 kcal/kg of “desirable” body weight, while a very-low-caloric diet provides ≤10 kcal/kg of desirable weight intake (28).
Low-carbohydrate/lipid-rich diets have been proposed and popularized as KDs. There is no consensus on a definition of KDs: in general, fat intake accounts for >75% of energy intake. The traditional 4:1 ratio is composed, in total calories, of 90% lipids, 8% proteins, and 2% carbohydrates, respectively (26, 27). Less-strict KDs have recently emerged, with ratios of 3:1 and even 2:1, and thus many current “KDs” often contain more protein. Thus, some KDs mimic the so-called Atkins diet, which did not restrict consumption of calories or proteins and was historically tested for intractable epilepsy (29), or can contain more carbohydrates due to a high proportion of medium chain triglycerides, which promote liver ketone body synthesis (27). In cancer research, isocaloric KDs are mostly used in order to maintain weight, which is essential for cancer patients undergoing therapy, and should be distinguished from nonisocaloric KDs that combine this strategy with moderate CR.
Protein restriction (PR) is defined by a reduction in protein intake, representing <12.5% of total calories without CR (14). It should also be considered that, beyond their raw caloric value, dietary proteins are also a source of essential (not synthesized by eukaryotic organisms) and conditionally essential amino acids, whose synthesis can be limited under special pathophysiological conditions: an imbalance in the pool of amino acids absorbed through diet may have important metabolic implications in protein synthesis. For this reason, a dietary strategy based on selective amino acid deprivation may _target specific metabolic patterns that are impaired in cancer cells (30).
Interplay of nutritional state with radiation sensitivity
Radiation induces oxidative stress by increasing ROS production, most notably superoxide ion released at the level of complex I and III of the respiratory chain. Initially, superoxide probably damages mitochondrial DNA, which is located in proximity of the respiratory chain and is not protected by histone proteins, as opposed to nuclear DNA (31). It is noteworthy that manganese superoxide dismutase (MnSOD)—converting superoxide to hydrogen peroxide, a less toxic compound—is located in mitochondria (31), suggesting that mitochondrial “dysfunction” (32, 33) has a major role in carcinogenesis through loss of mitochondria (34) and/or altered metabolism (35).
Delivery of an effective RT dose is limited by its toxicity on healthy tissues, especially on proliferating cells (bone marrow, gastrointestinal, hair follicles, and heart cells). Hence, it is of primary importance to selectively increase tolerance and recovery of normal cells. Unfortunately, radioprotective properties of natural antioxidants (e.g., glutathione, vitamin E, and analogs) or synthetic compounds (amifostine) are limited or questioned (36). Interestingly, SF, CR, and a KD could exert radioprotective effects because healthy cells would more efficiently adapt to glucose starvation than cancer cells, which led to a concept of a “differential stress resistance” (37–39) (Supplemental Figure 1).
The rational explanation supporting differential stress resistance is that metabolism of healthy cells is censored by suppressive control checkpoints [Rb, p53, p21, phosphatase and tensin homolog (PTEN), sirtuin-3 (SIRT3)], and is thus more flexible than cancer cell metabolism, which lacks censoring mechanisms (40, 41).
In healthy cells, glucose starvation induces a downregulation of the PI3K/AKT/mTOR proliferative pathway, resulting in the inhibition of the Warburg effect (42, 43), while activation of AMP-kinase (AMPK), the key energy sensor, activates fatty acid oxidation (FAO) and inhibits glycolysis (44). Of note, FAO is the most efficient catabolic pathway generating ATP and NAD. Both molecules, sustaining cell survival and repair, are regulated by p53, p21, the protein kinase forkhead box protein O3 (FOXO3), and the NAD+-dependent poly[ADP-ribose]polymerase 1 (PARP1) (45–47). Additionally, ROS neutralization is promoted by NAD+-dependent SIRT3, which upregulates MnSOD2 and also counteracts the Warburg effect (48–50).
Glucose-starved cancer cells may escape these regulatory checkpoints, particularly when driven by strong oncogenic signals such as RAS/RAF (rapidly accelerated fibrosarcoma), insulin-like growth factor I (IGF-I) axis, mitogen-activated protein kinase (MAPK), and c-Myc dictating an anabolic metabolism and forcing cells to replicate (51, 52), especially as suppressive controls are defectives. This can have important consequences for the metabolic assets of tumor cell, due to the dual-faceted activity of key regulator enzymes: for example, AMPK may shift from a profile characterized by a dominant activation β-subunit of AMPK, which promotes glycolysis and inhibits oxidative phosphorylation (OXPHOS) (44), to a prevalent activation of the α-subunit (53,54) under glucose starvation, thus forcing cancer cells to increase OXPHOS and generating more ATP with an increase in ROS production.
Similarly to SF and CR, KDs induce chronic glucose starvation since, in contrast with normal cells found in healthy tissues (in particular, the brain, heart, and muscle), cancer cells cannot catabolize either exogenous (supplied by dietary intake) fatty acids or endogenous ketone bodies (derived from FAO and released by the liver) to produce energy (55,56) because they lack catabolizing mitochondrial enzymes [i.e., 3β-hydroxybutyrate dehydrogenase (3β-OHBD) and succinyl-CoA:3-ketoacid-coenzyme A transferase (SCOT)] (56).
Concerning the interest of protein/amino acid restriction, it must be noted that biosynthesis and proliferation of various cancer cells lines can be supported by other nutrients than glucose (i.e., glutamine), especially in case of glucose starvation (57,58). Starvation in amino acids can inhibit tumor proliferation, as shown by arginine (59,60), serine, glycine (61), or methionine deprivation (62). Interestingly, cisplatin-resistant cells appear to be very sensitive to glutamine starvation (63). Arginine deprivation can counteract cancer development because the molecule is a precursor of polyamines, NO, and proline. An arginine diet could be effective in many cancers knowing that arginine-succinate synthetase 1 (ASS1) is commonly reduced or lost in liver cancer, metastatic melanoma, renal cell carcinoma, platinum refractory ovarian tumor, and in almost 90% of sarcomas regardless of their subtypes (60). In sarcoma, arginine deprivation with pegylated arginine deiminase induces cancer cell death if it is associated with chloroquine, an inhibitor of autophagy (60).
Current Status of Knowledge
Search methodology
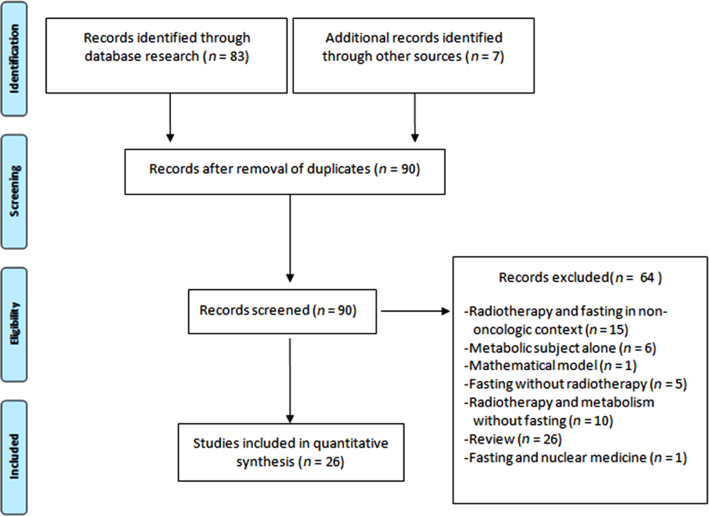
A literary search was performed in PubMed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (64) using the following keywords: fasting, caloric restriction, ketogenic diet, protein restriction, amino-acid restriction, Warburg effect, and cancer and radiation. Evaluation of appropriateness was independently carried out by 2-author teams with expertise in radiation oncology and tumor metabolism (ML and MA, PI and LO). In case of inconsistency or disagreement, a final decision was formulated with a third author team (JT and PF). Thus, we identified 484 potentially eligible articles and checked their references for additional articles. Using this manual ad hoc checking, we found 7 additional articles. The progression of this methodology is summarized in Figure 1. After duplicates were removed, 90 articles remained. Among them, according to a consensus of all authors, we finally identified 26 eligible articles that reported data on RT and diets. In total, we found 9 original studies on cultured cells, 8 original studies in murine models, and 9 clinical studies including 2 case reports.
FIGURE 1.
Flowchart for the literary search according to PRISMA. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Preclinical experiments in SF and CR
In vitro experiments showed that near-complete SF increases cancer cell sensitivity to RT in various cancer cell lines (65,66). In a preclinical model of SF, hepatocellular carcinoma (HepG2), and hepatoblastoma cells (HuH6-clone5) were cultured in serum-free media for 6 to 24 h, showing increased sensitivity to RT (range: 0–10 Gy) correlated with activation of mammalian _target of rapamycin complex 1 (mTORC1), a critical pathway involved in ROS-mediated cell damage detection [via ataxia telangiectasia mutated (ATM)] and repair (via modulation of autophagy) (65). Glucose starvation for 24 h increased DNA damage and double-strand-break DNA lesions induced by RT in the lung adenocarcinoma A549 cells and in the head and neck squamous cancer FaDu cells, while normal HSF7 fibroblasts were not affected by glucose starvation (66). Another model of glucose starvation, induced by inhibition of glucose uptake with 2-deoxyglucose, also increased sensitivity to radiation (2 Gy) in radio-resistant rSCC-61 head and neck cancer cells (67).
In vivo experiments studying short-term SF and CR associated with RT on different mice models of cancers are reported in Table 1 (68–72). In summary, a CR of 30% increased tumor regression or delayed occurrence of metastases in 3 animal models of mammary tumors, including 2 models of triple-negative breast cancer (TNBC) (71, 72). The decrease in metastatic dissemination of tumor cells correlated with the downregulation of IGF-I receptor (IGF-IR), and PI3K/mTOR pathways, as well as the inhibition of the microRNA-17/20a cluster regulating expression of extracellular membrane proteins (72, 73). More pronounced caloric starvation (70% CR), coupled with low-dose irradiation (0.04 Gy) induced regression of spontaneous mammary cancers in C3H/He mice, associated with massive infiltration of cytotoxic CD8+ T cells (69). SF for 48 h, prior to RT and temozolomide (TMZ) increased survival of mice bearing glioma tumors implanted subcutaneously or in the brain (68). Remarkably, in this study, SF as a single therapy significantly increased survival, decreased the size of subcutaneous glioma, and reinforced the effects of TMZ in intracranial tumors. A significant reduction in circulating blood glucose and IGF-I concentrations levels was also noticed.
TABLE 1.
Short-term feed deprivation and caloric restriction associated with RT in mouse models of cancers: effects on tumor volume and survival1
| Authors, year (ref) | Cancer model | Therapeutic protocol | Oncological effects |
|---|---|---|---|
| Kharazi et al., 1994 (69) | Female C3H/He mice, spontaneous mammary tumor | 70% severe CR for 1 mo;RT, low dose: 0.04 Gy source, 3 times/wk for 4 wk | 70% RTV under CR + RT; T cells CD8+ in tumors; no effect with RT or CT alone |
| Safdie et al., 2012 (68) | GliomaGL26 cells, subcutaneous or intracranial models | SF 48 h before TMZ ± RT : 7.5, 5, 2.5 Gy, at days 1, 15, and 22 | DTG and IS |
| Saleh et al., 2013 (71) | TNBC (4T1 and 67NR cells) | 30% CR + RT at initial burden; 6 Gy for 67NR cells, 8 Gy for 4T1 cells. | RTV |
| Simone et al., 2016 (72) | TNBC (4T1 cells) | 30% CR at initial burden+ RT: 8 Gy | Lung metastases delayed (occurrence and number), IS |
1CR, caloric restriction; CT, chemotherapy; DTG, delaying tumor growth; IS, increased survival; KD, ketogenic diet; ref, reference; RT, radiotherapy; RTV, regression of tumor volume; SF, short fasting; TMZ, temozolomide; TNBC, triple-negative breast cancer.
We found no clear evidence from clinical studies evaluating RT with SF or CR. One clinical trial currently is evaluating the benefit of CR (∼25%) in patients with localized breast cancer undergoing surgery and RT (clinicaltrial.gov, NCT01819233): results are not yet available.
Preclinical experiments in KDs
In vitro, the administration of ketone body 3β-hydroxybutyrate (3β-OHB) at 3 mM concentration failed to induce additional inhibitory effects on the proliferation of 7 human breast cancer cells lines (BT20, BT474, HBL100, MCF-7, MDA-MB 231, MDA-MB 468, and T47D) exposed to various doses of RT (0, 2, 4, 6, and 8 Gy) in association with different cytotoxic agents (carboplatin, epirubicin, paclitaxel) and cultured in 5 mM glucose medium in response (74).
In vivo experiments (Table 2) (75, 76) showed slower tumor progression as well as increased survival in mice grafted with high-grade glioma, lung, or pancreatic cancer cells, when mice received a KD in association with RT (75–77).
TABLE 2.
The effects of a KD associated with RT in mouse cancer models1
| Authors, year (ref) | Type of cancer model | Therapeutic protocol | KD, % of lipids/carbohydrates/proteins | RT administration | Oncological effects |
|---|---|---|---|---|---|
| Abdelwahab et al., 2012 (75) | Glioma (GL261-luc2 cells); intracranial implantation in albino mice | KD + RT vs SD + RT | 72/3/15 | Whole-brain irradiation: 2 × 4 Gy | RTV |
| Allen et al., 2013 (76) | Lung cancer (NCI-H292 and A549 cells) | KD ± RT ± CT (carboplatin) | 90/1.6/8.4 | 34 times 1.8 Gy, 6 times 2 Gy; CON group: 2 times 6 Gy | RTV and ISKD alone; no effect |
| Zahra et al., 2017 (77) | Pancreas cancer (Mia-Paca-2 cells) | KD + RT | 69/2.8/14.3 | 12 Gy: 6 × 2 Gy | RTV and IS |
1CON group, control group; CT, chemotherapy; IS, increasing survival; KD, ketogenic diet; ref, reference; RT, radiotherapy (conventionally fractionated, 1.8–2 Gy; or hypofractionated (6 Gy) radiation as well as conventionally fractionated radiation); RTV, regression of tumor volume; SD, standard diet.
Human noncontrolled/single-arm pilot studies and 2 case reports (in total, n = 77 patients) reporting RT and KD associations are presented in Table 3 (70, 77–84). Isocaloric KDs were used in the 5 pilot studies, while a nonisocaloric approach combining KD + CR (660–900 kcal/d) was applied to the 2 case reports. Briefly, in these small cohorts of miscellaneous tumors (including glioma, breast, prostate, rectum, or small cell lung cancers), stabilization or regression of cancers was often reported. Concerning the 2 individual case reports, it was proposed that the rapid regression of glioma tumors after subtotal resection could be partially ascribed to the strict diet regimen (3-d SF followed by severe CR reducing the caloric intake to ∼60%, then a KD for several months), which was administered during the course of RT and TMZ (79, 83).
TABLE 3.
Noncontrolled studies reporting results of RT associated with a KD in cancer patients1
| Authors, year (ref) | Cancer types | Therapeutic protocol | KD, % of lipids/carbohydrates/proteins | Radiotherapy | Effects |
|---|---|---|---|---|---|
| Nebeling et al., 1995 (78) | Astrocytoma, advanced stage | RT + CT + KD | 70/20/10 | 36 Gy on neuroaxis, 54 Gy on posterior fossa | Tumor response with possible increasing OS |
| Zuccoli et al., 2010 (79) | Glioblastoma | CR (660 kcal/d) + RT + TMZ + KD | 75/15/10 | 60 Gy in 30 fractions of 2 Gy | TR, recurrence after diet suspension5 TP |
| Champ et al., 2014 (80) | Glioblastoma multiforme (n = 6) | RT + TMZ + KD | 77/15/8 | 60 Gy in 30 fractions of 2 Gy | 1 complete response |
| Klement et al., 2016 (81) | Breast, prostate, SCLC, and 2 rectal adenocarcinomas (n = 6) | RT + CT + KD | 73/12/5 | ST | 1 TP (SCLC) and 5 TR |
| Kato et al., 2016 (82) | Invasive rectal cancers | RT + KD | 40% kcal fat and CHO <100 g/d | ST | Reduced risk of cancer specific death? |
| Zahra et al., 2017 (77) | NSCLC (n = 7), pancreas (n = 2) | RT + CT + KD | 90/2/8 | ST | No difference in OS and PFS |
| Elsakka et al., 2018 (83) | Glioblastoma multiforme | RT + TMZ + CR (900 kcal/d) + KD + HOBT + MIX | 70/15/15 | 30 times 2 Gy | TR |
| van der Louw et al., 2019 (84) | Intrinsic Pontine glioma (n = 3) | RT + CT (TMZ or GCB) + KD 4:1 (for 3 mo) | Ketone levels >3 mmol/L KD with MCT emulsions | Fractionated RT up to 60 Gy | 3 patients died |
| Klement et al., 2019 (70) | Colorectal, breast, head, and neck cancers (n = 81): 20 KD vs. 61 control diet | SF + KD + RT ± CT | KD during RT: MCT + 10 g EAAs or full KD + 10 g EAAs | RT, RT + CT | Differential effects on weight fat and lean mass |
1CHO, carbohydrate; CR, caloric restriction; CT, chemotherapy; EAA, essential amino acid; GCB, gemcitabine; HOBT, hyperbaric oxygen therapy; KD, ketogenic diet; MCT, medium-chain triglyceride; MIX, metformin, methylfolate, chloroquine, epigallocatechin gallate, and levetiracetam; NSCLC, non–small cell lung cancer; OS, overall survival; PFS, progression-free survival; ref, reference; RT, radiotherapy; SCLC, small cell lung cancer; SF, short fasting; ST, standard treatment; TMZ, temozolomide; TP, tumor progression; TR, tumor response.
In both cases, no steroid medication was administered, and weight loss was maintained within 20% of baseline. In 1 case, despite initial major and rapid regression, a recurrence was observed at 9 mo, possibly in correspondence with suspension of the KD (79). In the second case, the patient ceased the KD at 9 mo and was still in good health at 20 mo, with a small, clinically stable residual disease (83).
As reported in Table 4, we list 15 clinical randomized trials testing RT with a KD. Results are not yet available.
TABLE 4.
Current clinical trials associating a KD and RT1
| Study number | Study | Protocols |
|---|---|---|
| NCT01975766 | KD phase 1 in head and neck cancer | RT + CT ± KD |
| ACTRN12614001056684 | Pilot study evaluating progression-free survival in glioma cancers under standard treatment (CT + RT) associated with KD | RT + CT ± KD |
| NCT01092247 | Effect of KD, CR, and IF cancer recurrence and progression (ARTZI 2017) | RT ± KD + CR and IF |
| NCT01754350 | KD, CR, and IF during re-irradiation of recurrent GBM (ERG02) | RT ± KD + CR and IF |
| NCT01819233 | CR in breast cancer undergoing surgery and RT | RT + CT + CR 25% |
| NCT02046187 | KD as adjunctive treatment of RT and CT in newly diagnosed glioma | RT = CT ± KD |
| NCT02302235 | KD as adjunctive treatment of RT and CT in glioma | RT + CT ± KD |
| NCT02149459 | Metabolic manipulation combined with RT as treatment of recurrent brain tumors (smc0712–13) | RT + KD + metformin |
| NCT02516501 | Impact on body composition of KD during RT (KETOCOMP) | RT ± KD |
| NCT03278249 | Feasibility study of modified Atkins KD in treatment of newly diagnosed glioma | RT + CT ± KD |
| NCT01419483 | KD with concurrent chemoradiation in pancreatic cancer | RT + CT + KD |
| NCT01419587 | KD with concurrent chemoradiation in non–small cell lung cancer. | RT + CT + KD |
| NCT01754350 | CR with IF and KD with concurrent RT in recurrent glioma | RT + CR + IF + KD |
| NCT02302235 | KD as adjunctive treatment of RT and CT in glioma | RT + CT ± KD |
| NCT02149459 | Metabolic manipulation combined with RT as treatment of recurrent brain tumors (smc0712–13) | RT + KD + metformin |
1CT, chemotherapy; CR, caloric restriction; IF, intermittent fasting; KD, ketogenic diet; RT, radiotherapy.
Preclinical experiments in protein/amino acid restriction
Only in vitro models of amino acid restriction were identified in our search, while no studies of PR were identified. Arginine starvation induced massive apoptosis in 4 human epithelial cancer cell lines in 2D monolayer and 3D spheroid cultures and was remarkably efficient in association with RT (85). Pretreatment of cancer cells with arginase, an enzyme involved in arginine degradation, significantly enhanced the response to RT. The sensitivity to RT was reinforced by a low concentration of canavanine, a toxic arginine analog (86).
In a preclinical study, methionine starvation altered the metabolomic profile and significantly reduced tumor growth after a focal 20-Gy irradiation, in a mouse model of constitutively chemo- and radio-resistant human soft tissue sarcoma with mutated, Kirsten rat sarcoma (KRAS), and p53-deficient cells (62). We found no other animal model or human clinical trials studying RT in association with either PR or amino acid restriction. Of note, despite 4 trials investigating PR in cancer in Clinicaltrials.gov, none address the question of the combination with RT.
Dietary interventions in association with RT
As shown by in vitro and in vivo studies (Table 1), SF potentially increases the radiosensitivity of glioma tumors (68), while 30% to 70% CR increased tumor sensitivity to RT + CT in several mammary cancer models (including TNBC) (71, 72). The increasing regression of tumors after short SF or CR associated with RT with or without CT has been correlated with the downregulation of the IGF-I/PI3K/mTOR pathway. Indeed, decreased glucose and IGF-I serum concentrations were observed during SF + RT (68), as well as reduced concentrations of IGF-IR (72) and downregulation of mTOR (71). This suppressive effect of CR or short SF on the IGF-I/PI3K/mTOR axis is extremely relevant considering the key role of this pathway in promoting the Warburg effect and tumor growth (87–89). It is noteworthy that IGF-IR is not counteracted by a dysfunctional or mutated p53 (87, 90) and is frequently overexpressed in various aggressive cancers (91). Interestingly, CR has been found, in a murine model, to reduce the risk of late occurrence of cancer after RT (92). This preventive role could rely on epigenetic processes and activation of immune surveillance. Concerning this latter aspect, SF also enhances the immune cytotoxic response against cancer cells (Table 1): a severe 70% CR during 1 mo associated with low-dose RT was shown to induce a massive cytotoxic response in a mammary cancer mouse model, albeit the uncombined treatment was not sufficient to induce this effect (69). Furthermore, intermittent 24-h SF for 2–3 wk increased tolerance to whole-body irradiation in mice and was associated with a better recovery of the leucocyte blood cell count following a sublethal dose of 5.26 Gy (93). Because cancer cells compete with lymphocytes and divert glucose for their own use (94), SF or CR associated with RT might likely activate an acute immune response by increasing glycolysis in CD8+ T cells, while glycolysis in cancer cells is inhibited. Likewise, activation of glycolysis in effector T cells leads to inactivation of programmed death 1 (PD-1) ligand (PD-L1), resulting in their expansion and activation with release of IFN-γ (95).
In summary, preclinical experiments support the hypothesis that short SF and CR promote effectiveness of RT by increasing cytotoxic stress, acute inflammation, and immune response; moreover, inhibition of the IGF-IR signaling pathway inactivates cancer cell proliferation and supports recovery of healthy cells.
Focusing on a KD, an increased response to RT in various animal models has been reported, consisting of delayed tumor progression, reduced occurrence of lung metastases, and prolonged survival, as seen in Table 2. In patients (Table 3), several studies suggested a possible benefit of KDs on tumor response.
It is noteworthy that, similarly to SF/CR, a KD may increase the immune response as shown in murine models of glioma by decreasing expression of immune inhibitory receptors PD-1 and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) as well as their inhibitory ligands (96). In accordance, immunosuppressive regulatory T cells and myeloid-derived suppressor cells (MDSCs) were depleted in another study (97).
In addition to these possible tumor-directed effects of a KD which could reinforce the efficiency of conventional treatment, especially in brain tumors (33), several studies have pointed out other beneficial effects of KDs on physical and mental condition. A KD is often well accepted and tolerated (98), in particular in children with brain tumor (78,84), despite frequent fatigue, constipation, and weight loss. An improvement in quality of life can be expected (99) and weight loss is likely dependent on the duration of the KD and extent of CR: weight decreased by a mean of 4% (0.0–6.1%) in 10 cancer patients receiving a KD with 35% ± 6% CR (100).
A better preservation of lean mass could be expected, since weight loss should mainly occur at the expense of body fat (26, 27). In a recent interim analysis reported by the KETOCOMP group, studying the benefit of a KD on RT in patients with rectal and breast cancers, 20 patients receiving a KD showed a loss of 0.5 and 0.4 kg fat mass/wk, with no significant changes in fat-free and skeletal muscle mass (70). This preservation of lean mass seems in line with the physiological regulation promoting gluconeogenesis in case of starvation, because this pathway is primarily sustained by glycerol provided by lipolysis and not by amino acids derived from proteolysis (101); this reduction in amino acid consumption delays proteolysis and loss of muscle resulting in sarcopenia, a process favored by a low baseline BMI reflecting poor fat reserves (102–104).
With regard to protein/amino acid restriction, the evidence in favor of a radiosensitizing effect of these interventions according to our analysis of the available literature is limited to single amino acid deprivation: for this reason, the impact of deprivation of simple amino acids cannot be differentiated from that caused by a reduction in the intake of whole proteins. This has important implication, since whole protein–restricted diets could be easier to prepare than diets based on single amino acids, considering, for example, that a vegan diet is low in methionine (105). Other than its exclusive metabolic implications, PR may be involved in modulation of immune antitumor response. Very recently, a low-protein (<5%) isocaloric diet reduced tumor growth in 3 independent mouse cancer models (lymphoma, melanoma, and colon cancer); the anticancer effect of this moderate-protein starvation was mediated by activation of CD8+ T-cell immune response. In contrast, a low-carbohydrate diet had no effect in these mouse models (106).
Controversies
It has been reported that ketone bodies may be used by tumor cells as a substrate for energy metabolism as shown by in vitro and in vivo experiments (107, 108). For instance, 3β-OHB did not influence proliferation and response to CT and RT of several breast cancer cell lines cultured in low-glucose medium (5 mM) (74), but accelerated tumor growth in an acute myeloid leukemia xenograft model (109) as well as some breast cancer models (110, 111). In mice bearing MMTV-NEU-NT mammary tumors, 3β-OHB increased ATP production in cancer cells (measured by spectrometer) and promoted tumor growth, while demonstrating no effect on histone acetylation (108). In fact, very few studies have been conducted on cell lines supporting the “paradigm” that cancer cells lack 3β-OHBD and SCOT (55, 112–114). Several authors reported the capability of cancer cells to utilize fatty acids, especially when they grow in rich adipocyte tissues or environment (115–118). Tisdale and Brennan (55) studied 10 murine cell lines, including 5 hematopoietic cells lines: 1 sarcoma cell line, 1 carcinosarcoma cell line, 1 “rat” cell line, and 2 bladder cell lines. While SCOT activity was reduced in these tumor cells in comparison with normal tissues, 3β-OHBD activity levels were quite similar. Moreover, in 4 cells lines cultured with 2 mM of 3β-OHBD for 7 d, the decrease in 3β-OHBD over this period ranged from 14% to 44%, thus demonstrating significant consumption of the ketone bodies.
It could be inferred that specific brain tumors (e.g., astrocytoma, schwannoma, and craniopharyngioma) may have significantly lower concentrations of enzymes catabolizing ketone bodies (in particular, SCOT), in comparison with normal brain; however, a panel of 7 glioblastoma exhibited a wide range of enzymatic activities (112). More recently, gene expression of 3β-OHBD and SCOT has been found to be lower in malignant astrocytoma (CT-2A) and human malignant glioma (U87-MG) cells lines implanted in the brain of mice (113), as well as in human neuroblastoma (SK-N-AS) cell lines (112–114) in comparison with normal brain. It is noteworthy that, in 2 patients with glioblastoma showing little benefit from a KD, in both cases tumors expressed mitochondrial 3β-OHBD and SCOT (119). Furthermore, even if SCOT is deficient, acetoacetate could promote tumor growth as shown in mice bearing human melanoma xenografts with BRAF V600E expression, the binding of acetoacetate to BRAF protein promoting growth (107).
Of note, the small numbers of patients in these cohorts and the absence of control groups hinder any definitive conclusions. Furthermore, in 3 studies, a KD was not the only variable associated with response to RT but was frequently combined with other therapeutic measures such as chemotherapy (70,77–80), SF (70) or CR (79, 83). With regard to the latter, it should be noted that unintended CR may occur in patients receiving an isocaloric KD (100), resulting in additional confounding bias.
Hence, the presence of multiple confounders is a serious limitation in defining the individual impact of a KD in retrospective experiments, as proposed by some authors (120), while results from 15 clinical randomized trials testing a KD and RT are not yet available (Table 4).
It should be pointed out that numerous cancer cells are not inherently glycolytic but rely on a predominant oxidative metabolism or an intermediate functioning (121, 122). Thus, as remarked by Rodrigues et al. (108), the “butyrate paradox” is likely related to the capability of 3β-OHBD acting as an energy source in cells supported by an oxidative metabolism, and as an epigenetic factor inhibiting cancer growth in cells relying on the Warburg effect (123). Under glucose deprivation (0.5-mM concentration) cancer cells carrying KRAS and BRAF mutations can also increase the expression of glucose transporter type 1 (GLUT1), a carrier that has a high avidity for glucose (124). Thus, stress conditions may select resistant cancer cells capable of adapting to changes in their microenvironment by modifying their metabolism, epigenome, and genomes.
Diets based on a single amino acid may have dual effects: for example, a protein-deficient diet, in particular with reduced methionine, can promote hepatocarcinogenesis in animals (125, 126), while arginine supplementation antagonizes both in vitro and in vivo the malignant transformation of mammary epithelial cells (59). Of note, arginine supplementation could also improve the performance status and Karnofsky index of patients with esophageal cancers (127) and reduces (in association with glutamine and fish oil) the incidence of severe hematologic toxicities occurring during CT and RT (128).
Concluding Remarks
In conclusion, our literature review offers preliminary evidences that SF before RT and CR during RT sessions may improve tumor response to radiation. Repeated sessions may increase the efficiency of RT administration and might exert a radioprotective effect on healthy tissues. This nutritional strategy might be of interest for tumors displaying high glucose uptake on PET scan, a feature associated with poor survival that may be related to Warburg metabolism functioning (22, 23).
Other interventions, such as KDs, may be more hazardous, considering the contradictory results of preclinical studies, and some authors have advised against their use in cancer patients (120). Even if preclinical studies and limited clinical experiments argued in favor of a possible beneficial synergistic effect of KDs and RT in high-grade cancers (in particular for TNBC and brain tumors) (27, 33, 98, 119), more robust data are needed.
Only evidence-based data from randomized clinical trials can evaluate the impact of dietary interventions on response to cancer treatments (129). It should be pointed out that, even if the majority of animal studies (∼70%) provide evidence of an antitumor effect of KDs (130), preclinical models give often discordant results and do not reflect the clinical situation, since the metabolic rate in mice is 7-fold higher than in humans (131).
Human trials should strictly define daily caloric intake, composition of diets, placebo diets, biological parameters assessing glucose starvation and ketosis (132), as well as prespecified criteria for the evaluation of tumor response. To assess which nutritional strategies should be favored, a deeper knowledge of the specific biological vulnerabilities of each cancer type should be obtained. For that purpose, assessment of various proteins (IGF-I) and metabolite profiles on serum analyses (133) and expression analysis of enzymes and membrane transporters on tumor biopsies (134) could be performed to identify the metabolic profile involved in a specific clinical situation (20, 135, 136).
Finally, modulation of nutrition during RT could be a simple and medically economical and inexpensive method that may deserve to be tested to improve efficiency of RT by exploiting increased radiosensitivity of tumor cells while reducing radiation-related injury to healthy tissues.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge Gilles Girault (Centre Francois Baclesse, Caen, France) for his technical assistance in the research of references. The authors’ responsibilities were as follows—PI and JT: designed the manuscript; PI and LO: wrote the manuscript; JT: entered references; ML, PF, JO, and MA: prepared the figures or commented on the text; LF: contributed to comments, literature review, and editing; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this work.
Author disclosures: The authors report no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AKT, protein kinase B; AMPK, AMP-activated protein kinase; CR, caloric restriction; CT, chemotherapy; FAO, fatty acid oxidation; HIF-1, hypoxia-inducible factor 1; IGF-I, insulin-like growth factor I; IGF-IR, insulin-like growth factor I receptor; KD, ketogenic diet; MnSOD, manganese superoxide dismutase; mTOR, mammalian _target of rapamycin; OXPHOS, oxidative phosphorylation; PD-1, programmed death 1; PI3K, phosphatidylinositol 3-kinase; PR, protein restriction; ROS, reactive oxygen species; RT, radiotherapy; SCOT, succinyl-coenzyme A-3-ketoacid-CoA transferase; SF, short fasting; SIRT3, sirtuin-3; TBNC, triple-negative breast cancer; TCA, tricarboxylic acid cycle; TMZ, temozolomide; 3β-OHBD, 3β-hydroxybutyrate dehydrogenase; 3β-OHB, 3β-hydroxybutyrate; 18FDG-PET, 18F-fluorodeoxyglucose–positron emission tomography.
References
- 1. Sonveaux P. ROS and radiotherapy: more we care. Onco_target. 2017;8:35482–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat. 2011;14:191–201. [DOI] [PubMed] [Google Scholar]
- 4. Stepien K, Ostrowski RP, Matyja E. Hyperbaric oxygen as an adjunctive therapy in treatment of malignancies, including brain tumours. Med Oncol. 2016;33:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett MH, Feldmeier J, Smee R, Milross C. Hyperbaric oxygenation for tumour sensitisation to radiotherapy. Cochrane Database Syst Rev. 2018;4:CD005007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee SL, Rouhi P, Dahl Jensen L, Zhang D, Ji H, Hauptmann G, Ingham P, Cao Y. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci. 2009;106:19485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, Lindeløv B, Jørgensen K. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma: results of the Danish Head and Neck Cancer Study (DAHANCA) protocol 5–85. Radiother Oncol. 1998;46:135–46. [DOI] [PubMed] [Google Scholar]
- 8. Hassan Metwally MA, Ali R, Kuddu M, Shouman T, Strojan P, Iqbal K, Prasad R, Grau C, Overgaard J. IAEA-HypoX: a randomized multicenter study of the hypoxic radiosensitizer nimorazole concomitant with accelerated radiotherapy in head and neck squamous cell carcinoma. Radiother Oncol. 2015;11:15–20. [DOI] [PubMed] [Google Scholar]
- 9. Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A et al.. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:124ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li ZQ, Zou L, Liu TR, Yang AK. Prognostic value of body mass index before treatment for laryngeal squamous cell carcinoma. Cancer Biol Med. 2015;12:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsang NM, Pai PC, Chuang CC, Chuang WC, Tseng CK, Chang KP, Yen TC, Lin JD, Chang JT. Overweight and obesity predict better overall survival rates in cancer patients with distant metastases. Cancer Med. 2016;5(4):665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hou WH, Wang CW, Tsai CL, Hsu FM, Cheng JC. The ratio of weight loss to planning _target volume significantly impacts setup errors in nasopharyngeal cancer patients undergoing helical tomotherapy with daily megavoltage computed tomography. Radiol Oncol. 2016;50:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pili R, Fontana L.. Low-protein diet in cancer: ready for prime time?. Nat Rev Endocrinol. 2018;14:384–6. [DOI] [PubMed] [Google Scholar]
- 15. Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. [DOI] [PubMed] [Google Scholar]
- 16. Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–37. [DOI] [PubMed] [Google Scholar]
- 17. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells?. Drug Resist Updat. 2018;38:1–11. [DOI] [PubMed] [Google Scholar]
- 19. Icard P, Kafara P, Steyaert JM, Schwartz L, Lincet H. The metabolic cooperation between cells in solid cancer tumors. Biochim Biophys Acta. 2014;1846:216–25. [DOI] [PubMed] [Google Scholar]
- 20. Curry JM, Tuluc M, Whitaker-Menezes D, Ames JA, Anantharaman A, Butera A, Leiby B, Cognetti DM, Sotgia F, Lisanti MP et al.. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle. 2013;12:1371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leung E, Cairns RA, Chaudary N, Vellanki RN, Kalliomaki T, Moriyama EH, Mujcic H, Wilson BC, Wouters BG, Hill R et al.. Metabolic _targeting of HIF-dependent glycolysis reduces lactate, increases oxygen consumption and enhances response to high-dose single-fraction radiotherapy in hypoxic solid tumors. BMC Cancer. 2017;17:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Na F, Wang J, Li C, Deng L, Xue J, Lu Y. Primary tumor standardized uptake value measured on F18-fluorodeoxyglucose positron emission tomography is of prediction value for survival and local control in non-small-cell lung cancer receiving radiotherapy: meta-analysis. J Thorac Oncol. 2014;9:834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riester M, Xu Q, Moreira A, Zheng J, Michor F, Downey RJ. The Warburg effect: persistence of stem-cell metabolism in cancers as a failure of differentiation. Ann Oncol. 2018;29:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: an update. Ageing Res Rev. 2017;39:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klement RJ. Fasting, fats, and physics: combining ketogenic and radiation therapy against cancer. Complement Med Res. 2018;25:102–13. [DOI] [PubMed] [Google Scholar]
- 27. Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer—where do we stand?. Mol Metab. 2019;33:102–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Atkinson RL. Low and very low calorie diets. Med Clin North Am. 1989;73:203–15. [DOI] [PubMed] [Google Scholar]
- 29. Kossoff EH, Krauss GL, McGrogan JR, Freeman JM. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61:1789–91. [DOI] [PubMed] [Google Scholar]
- 30. Jeon H, Kim JH, Lee E, Jang YJ, Son JE, Kwon JY, Lim TG, Kim S, Park JH, Kim JE et al.. Methionine deprivation suppresses triple-negative breast cancer metastasis in vitro and in vivo. Onco_target. 2016;7(41):67223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kam WW, Banati RB. Effects of ionizing radiation on mitochondria. Free Radic Biol Med. 2013;65:607–19. [DOI] [PubMed] [Google Scholar]
- 32. John AP. Dysfunctional mitochondria, not oxygen insufficiency, cause cancer cells to produce inordinate amounts of lactic acid: the impact of this on the treatment of cancer. Med Hypotheses. 2001;57:429–31. [DOI] [PubMed] [Google Scholar]
- 33. Seyfried TN, Shelton L, Arismendi-Morillo G, Kalamian M, Elsakka A, Maroon J, Mukherjee P. Provocative question: should ketogenic metabolic therapy become the standard of care for glioblastoma?. Neurochem Res. 2019;44(10):2392–404. [DOI] [PubMed] [Google Scholar]
- 34. Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. [DOI] [PubMed] [Google Scholar]
- 35. Mathupala SP, Ko YH, Pedersen PL. The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochim Biophys Acta. 2010;1797:1225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sailo BL, Banik K, Padmavathi G, Javadi M, Bordoloi D, Kunnumakkara AB. Tocotrienols: the promising analogues of vitamin E for cancer therapeutics. Pharmacol Res. 2018;130:259–72. [DOI] [PubMed] [Google Scholar]
- 37. Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci. 2008;105:8215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Groot S, Pijl H, van der Hoeven JJM, Kroep JR. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–3. [DOI] [PubMed] [Google Scholar]
- 41. Haigis MC, Deng CX, Finley LW, Kim HS, Gius D. SIRT3 is a mitochondrial tumor suppressor: a scientific tale that connects aberrant cellular ROS, the Warburg effect, and carcinogenesis. Cancer Res. 2012;72:2468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dorff TB, Groshen S, Garcia A, Shah M, Tsao-Wei D, Pham H, Cheng CW, Brandhorst S, Cohen P, Wei M et al.. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jeon SM, Hay N.. The double-edged sword of AMPK signaling in cancer and its therapeutic implications. Arch Pharm Res. 2015;38:346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hashimoto N, Nagano H, Tanaka T. The role of tumor suppressor p53 in metabolism and energy regulation, and its implication in cancer and lifestyle-related diseases. Endocr J. 2019;66:485–96. [DOI] [PubMed] [Google Scholar]
- 46. Pradhan R, Kumar R, Shekhar S, Rai N, Ambashtha A, Banerjee J, Pathak M, Dwivedi SN, Dey S, Dey AB. Longevity and healthy ageing genes FOXO3A and SIRT3: serum protein marker and new road map to burst oxidative stress by Withania somnifera. Exp Gerontol. 2017;95:9–15. [DOI] [PubMed] [Google Scholar]
- 47. Sousa FG, Matuo R, Soares DG, Escargueil AE, Henriques JA, Larsen AK, Saffi J. PARPs and the DNA damage response. Carcinogenesis. 2012;33:1433–40. [DOI] [PubMed] [Google Scholar]
- 48. Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM et al.. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011;41:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–7. [DOI] [PubMed] [Google Scholar]
- 51. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. [DOI] [PubMed] [Google Scholar]
- 52. Icard P, Fournel L, Wu Z, Alifano M, Lincet H. Interconnection between metabolism and cell cycle in cancer. Trends Biochem Sci. 2019;44:490–501. [DOI] [PubMed] [Google Scholar]
- 53. Jiang D, Rusling JF.. Oxidation chemistry of DNA and p53 tumor suppressor gene. ChemistryOpen. 2019;8:252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiang M, Sun L, Isupov MN, Littlechild JA, Wu X, Wang Q, Wang Q, Yang W, Wu Y. Structural basis for the _target DNA recognition and binding by the MYB domain of phosphate starvation response 1. FEBS J. 2019;286:2809–21. [DOI] [PubMed] [Google Scholar]
- 55. Tisdale MJ, Brennan RA.. Loss of acetoacetate coenzyme A transferase activity in tumours of peripheral tissues. Br J Cancer. 1983;47:293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Branco AF, Ferreira A, Simões RF, Magalhães-Novais S, Zehowski C, Cope E, Silva AM, Pereira D, Sardão VA, Cunha-Oliveira T. Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur J Clin Invest. 2016;46:285–98. [DOI] [PubMed] [Google Scholar]
- 57. DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci. 2007;104:19345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Icard P, Wu Z, Alifano M, Fournel L. Gluconeogenesis of cancer cells is disrupted by citrate. Trends Cancer. 2019;5:265–6. [DOI] [PubMed] [Google Scholar]
- 59. Ren W, Li Y, Xia X, Guo W, Zhai T, Jin Y, Che Y, Gao H, Duan X, Ma H et al.. Arginine inhibits the malignant transformation induced by interferon-gamma through the NF-kappaB-GCN2/eIF2alpha signaling pathway in mammary epithelial cells in vitro and in vivo. Exp Cell Res. 2018;368:236–47. [DOI] [PubMed] [Google Scholar]
- 60. Bean GR, Kremer JC, Prudner BC, Schenone AD, Yao JC, Schultze MB, Chen DY, Tanas MR, Adkins DR, Bomalaski J et al.. A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death Dis. 2016;7:e2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJF, Mackay GM, Labuschagne CF, Gay D, Kruiswijk F et al.. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544:372–6. [DOI] [PubMed] [Google Scholar]
- 62. Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, Richie JP Jr, Ciccarella A, Calcagnotto A, Mikhael PG et al.. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Obrist F, Michels J, Durand S, Chery A, Pol J, Levesque S, Joseph A, Astesana V, Pietrocola F, Wu GS et al.. Metabolic vulnerability of cisplatin-resistant cancers. EMBO J. 2018;37:e98597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Murata Y, Uehara Y, Hosoi Y. Activation of mTORC1 under nutrient starvation conditions increases cellular radiosensitivity in human liver cancer cell lines, HepG2 and HuH6. Biochem Biophys Res Commun. 2015;468:684–90. [DOI] [PubMed] [Google Scholar]
- 66. Ampferl R, Rodemann HP, Mayer C, Hofling TTA, Dittmann K. Glucose starvation impairs DNA repair in tumour cells selectively by blocking histone acetylation. Radiother Oncol. 2018;126:465–70. [DOI] [PubMed] [Google Scholar]
- 67. Mims J, Bansal N, Bharadwaj MS, Chen X, Molina AJ, Tsang AW, Furdui CM. Energy metabolism in a matched model of radiation resistance for head and neck squamous cell cancer. Radiat Res. 2015;183:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, Conti PS, Chen TC, Longo VD. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One. 2012;7:e44603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kharazi AI, James SJ, Taylor JM, Lubinski JM, Nakamura LT, Makinodan T. Combined chronic low dose radiation-caloric restriction: a model for regression of spontaneous mammary tumor. Int J Radiat Oncol Biol Phys. 1994;28:641–7. [DOI] [PubMed] [Google Scholar]
- 70. Klement RJ, Schafer G, Sweeney R. A ketogenic diet exerts beneficial effects on body composition of cancer patients during radiotherapy: an interim analysis of the KETOCOMP study. J Trad Compl Med. 2019:1–8.. 10.1016/j.jtcme.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Saleh AD, Simone BA, Palazzo J, Savage JE, Sano Y, Dan T, Jin L, Champ CE, Zhao S, Lim M et al.. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle. 2013;12:1955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Simone BA, Dan T, Palagani A, Jin L, Han SY, Wright C, Savage JE, Gitman R, Lim MK, Palazzo J et al.. Caloric restriction coupled with radiation decreases metastatic burden in triple negative breast cancer. Cell Cycle. 2016;15:2265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jin L, Lim M, Zhao S, Sano Y, Simone BA, Savage JE, Wickstrom E, Camphausen K, Pestell RG, Simone NL. The metastatic potential of triple-negative breast cancer is decreased via caloric restriction-mediated reduction of the miR-17∼92 cluster. Breast Cancer Res Treat. 2014;146:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bartmann C, Janaki Raman SR, Flöter J, Schulze A, Bahlke K, Willingstorfer J, Strunz M, Wöckel A, Klement RJ, Kapp M et al.. Beta-hydroxybutyrate (3-OHB) can influence the energetic phenotype of breast cancer cells, but does not impact their proliferation and the response to chemotherapy or radiation. Cancer Metab. 2018;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, Scheck AC. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. 2012;7:e36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, Szweda LI, Smith BJ, Spitz DR, Fath MA. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res. 2013;19:3905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zahra A, Fath MA, Opat E, Mapuskar KA, Bhatia SK, Ma DC, Rodman SN III, Snyders TP, Chenard CA, Eichenberger-Gilmore JM et al.. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the University of Iowa experience of two phase 1 clinical trials. Radiat Res. 2017;187:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14:202–8. [DOI] [PubMed] [Google Scholar]
- 79. Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, Seyfried TN. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab (Lond). 2010;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Champ CE, Palmer JD, Volek JS, Werner-Wasik M, Andrews DW, Evans JJ, Glass J, Kim L, Shi W. _targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117:125–31. [DOI] [PubMed] [Google Scholar]
- 81. Klement RJ, Sweeney RA.. Impact of a ketogenic diet intervention during radiotherapy on body composition: I. Initial clinical experience with six prospectively studied patients. BMC Res Notes. 2016;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kato I, Dyson G, Snyder M, Kim HR, Severson RK. Differential effects of patient-related factors on the outcome of radiation therapy for rectal cancer. J Radiat Oncol. 2016;5:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Elsakka AMA, Bary MA, Abdelzaher E, Elnaggar M, Kalamian M, Mukherjee P, Seyfried TN. Management of glioblastoma multiforme in a patient treated with ketogenic metabolic therapy and modified standard of care: a 24-month follow-up. Front Nutr. 2018;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. van der Louw EJTM, Olieman JF, van den Bemt PMLA, Bromberg JEC, Oomen-de Hoop E, Neuteboom RF, Catsman-Berrevoets CE, Vincent AJPE. Ketogenic diet treatment as adjuvant to standard treatment of glioblastoma multiforme: a feasibility and safety study. Ther Adv Med Oncol. 2019;11:1758835919853958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vynnytska-Myronovska B, Bobak Y, Garbe Y, Dittfeld C, Stasyk O, Kunz-Schughart LA. Single amino acid arginine starvation efficiently sensitizes cancer cells to canavanine treatment and irradiation. Int J Cancer. 2012;130:2164–75. [DOI] [PubMed] [Google Scholar]
- 86. Vynnytska BO, Mayevska OM, Kurlishchuk YV, Bobak YP, Stasyk OV. Canavanine augments proapoptotic effects of arginine deprivation in cultured human cancer cells. Anticancer Drugs. 2011;22:148–57. [DOI] [PubMed] [Google Scholar]
- 87. Shi Y, Felley-Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA. Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin. BMC Cancer. 2012;12:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep. 2015;42:841–51. [DOI] [PubMed] [Google Scholar]
- 89. Bowers LW, Rossi EL, O'Flanagan CH, deGraffenried LA, Hursting SD. The role of the insulin/IGF system in cancer: lessons learned from clinical trials and the energy balance-cancer link. Front Endocrinol. 2015;6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Werner H, Sarfstein R, LeRoith D, Bruchim I. Insulin-like growth factor 1 signaling axis meets p53 genome protection pathways. Front Oncol. 2016;6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thariat J, Bensadoun RJ, Etienne-Grimaldi MC, Grall D, Penault-Llorca F, Dassonville O, Bertucci F, Cayre A, De Raucourt D, Geoffrois L et al.. Contrasted outcomes to gefitinib on tumoral IGF1R expression in head and neck cancer patients receiving postoperative chemoradiation (GORTEC trial 2004-02). Clin Cancer Res. 2012;18:5123–33. [DOI] [PubMed] [Google Scholar]
- 92. Shang Y, Kakinuma S, Yamauchi K, Morioka T, Kokubo T, Tani S, Takabatake T, Kataoka Y, Shimada Y. Cancer prevention by adult-onset calorie restriction after infant exposure to ionizing radiation in B6C3F1 male mice. Int J Cancer. 2014;135:1038–47. [DOI] [PubMed] [Google Scholar]
- 93. Kozubik A, Pospisil M. Adaptation to intermittent fasting as a factor modifying the radiation resistance of mice. Experientia. 1982;38:958–9. [DOI] [PubMed] [Google Scholar]
- 94. Sukumar M, Roychoudhuri R, Restifo NP. Nutrient competition: a new axis of tumor immunosuppression. Cell. 2015;162:1206–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ et al.. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. 2016;16:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Husain Z, Seth P, Sukhatme VP. Tumor-derived lactate and myeloid-derived suppressor cells: linking metabolism to cancer immunology. Oncoimmunology. 2013;2:e26383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rieger J, Bähr O, Maurer GD, Hattingen E, Franz K, Brucker D, Walenta S, Kämmerer U, Coy JF, Weller M et al.. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44:1843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schmidt M, Pfetzer N, Schwab M, Strauss I, Kammerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond). 2011;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fine EJ, Segal-Isaacson CJ, Feinman RD, Herszkopf S, Romano MC, Tomuta N, Bontempo AF, Negassa A, Sparano JA. _targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28:1028–35. [DOI] [PubMed] [Google Scholar]
- 101. Cahill GF Jr, Veech RL. Ketoacids? Good medicine?. Trans Am Clin Climatol Assoc. 2003;114:149–61.; discussion: 62–3. [PMC free article] [PubMed] [Google Scholar]
- 102. Collins S. A heart-adipose tissue connection in the regulation of energy metabolism. Nat Rev Endocrinol. 2014;10:157–63. [DOI] [PubMed] [Google Scholar]
- 103. Hervochon R, Bobbio A, Guinet C, Mansuet-Lupo A, Rabbat A, Régnard JF, Roche N, Damotte D, Iannelli A, Alifano M. Body mass index and total psoas area affect outcomes in patients undergoing pneumonectomy for cancer. Ann Thorac Surg. 2017;103:287–95. [DOI] [PubMed] [Google Scholar]
- 104. Icard P, Iannelli A, Lincet H, Alifano M. Sarcopenia in resected non-small cell lung cancer: let's move to patient-directed strategies. J Thorac Dis. 2018;10:S3138–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cavuoto P, Fenech MF. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat Rev. 2012;38:726–36. [DOI] [PubMed] [Google Scholar]
- 106. Rubio-Patiño C, Bossowski JP, De Donatis GM, Mondragón L, Villa E, Aira LE, Chiche J, Mhaidly R, Lebeaupin C, Marchetti S et al.. Low-protein diet induces IRE1alpha-dependent anticancer immunosurveillance. Cell Metab. 2018;27:828–42 e7. [DOI] [PubMed] [Google Scholar]
- 107. Xia S, Lin R, Jin L, Zhao L, Kang HB, Pan Y, Liu S, Qian G, Qian Z, Konstantakou E et al.. Prevention of dietary-fat-fueled ketogenesis attenuates BRAF V600E tumor growth. Cell Metab. 2017;25:358–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rodrigues LM, Uribe-Lewis S, Madhu B, Honess DJ, Stubbs M, Griffiths JR. The action of beta-hydroxybutyrate on the growth, metabolism and global histone H3 acetylation of spontaneous mouse mammary tumours: evidence of a beta-hydroxybutyrate paradox. Cancer Metab. 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, Amadiume SC, Goncalves MD, Hodakoski C, Lundquist MR et al.. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Martinez-Outschoorn UE, Balliet R, Lin Z, Whitaker-Menezes D, Birbe RC, Bombonati A, Pavlides S, Lamb R, Sneddon S, Howell A et al.. BRCA1 mutations drive oxidative stress and glycolysis in the tumor microenvironment: implications for breast cancer prevention with antioxidant therapies. Cell Cycle. 2012;11:4402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE et al.. Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fredericks M, Ramsey RB.. 3-Oxo acid coenzyme A transferase activity in brain and tumors of the nervous system. J Neurochem. 1978;31:1529–31. [DOI] [PubMed] [Google Scholar]
- 113. Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Skinner R, Trujillo A, Ma X, Beierle EA. Ketone bodies inhibit the viability of human neuroblastoma cells. J Pediatr Surg. 2009;44:212–6.; discussion: 6. [DOI] [PubMed] [Google Scholar]
- 115. Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B, Eyob H, Kajimura S, Tward A, Krings G et al.. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. 2016;22:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lengyel E, Makowski L, DiGiovanni J, Kolonin M. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4:374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nieman DC, Austin MD, Dew D, Utter AC. Validity of COSMED's quark CPET mixing chamber system in evaluating energy metabolism during aerobic exercise in healthy male adults. Res Sports Med. 2013;21:136–45. [DOI] [PubMed] [Google Scholar]
- 119. Schwartz K, Chang HT, Nikolai M, Pernicone J, Rhee S, Olson K, Kurniali PC, Hord NG, Noel M. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Erickson N, Boscheri A, Linke B, Huebner J. Systematic review: isocaloric ketogenic dietary regimes for cancer patients. Med Oncol. 2017;34(5):72. [DOI] [PubMed] [Google Scholar]
- 121. Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004;313:459–65. [DOI] [PubMed] [Google Scholar]
- 122. Peiris-Pages M, Martinez-Outschoorn UE, Pestell RG, Sotgia F, Lisanti MP. Cancer stem cell metabolism. Breast Cancer Res. 2016;18:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD et al.. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S, Zhou S et al.. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mikol YB, Hoover KL, Creasia D, Poirier LA. Hepatocarcinogenesis in rats fed methyl-deficient, amino acid-defined diets. Carcinogenesis. 1983;4:1619–29. [DOI] [PubMed] [Google Scholar]
- 126. Ghoshal AK, Farber E. The induction of resistant hepatocytes during initiation of liver carcinogenesis with chemicals in rats fed a choline deficient methionine low diet. Carcinogenesis. 1983;4:801–4. [DOI] [PubMed] [Google Scholar]
- 127. Vasson MP, Talvas J, Perche O, Dillies AF, Bachmann P, Pezet D, Achim AC, Pommier P, Racadot S, Weber A et al.. Immunonutrition improves functional capacities in head and neck and esophageal cancer patients undergoing radiochemotherapy: a randomized clinical trial. Clin Nutr. 2014;33:204–10. [DOI] [PubMed] [Google Scholar]
- 128. Chitapanarux I, Traisathit P, Chitapanarux T, Jiratrachu R, Chottaweesak P, Chakrabandhu S, Rasio W, Pisprasert V, Sripan P. Arginine, glutamine, and fish oil supplementation in cancer patients treated with concurrent chemoradiotherapy: a randomized control study. Curr Probl Cancer. 2019;44(1):100482. [DOI] [PubMed] [Google Scholar]
- 129. Caccialanza R, Cereda E, De Lorenzo F, Farina G, Pedrazzoli P; Group AIOM-SINPE-FAVO Working Group . To fast, or not to fast before chemotherapy, that is the question. BMC Cancer. 2018;18:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Klement RJ. Beneficial effects of ketogenic diets for cancer patients: a realist review with focus on evidence and confirmation. Med Oncol. 2017;34:132. [DOI] [PubMed] [Google Scholar]
- 131. Demetrius L. Of mice and men: when it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005;6(Spec No):S39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Miller VJ, Villamena FA, Volek JS. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab. 2018;2018:5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cheng Y, Yang X, Deng X, Zhang X, Li P, Tao J, Qin C, Wei J, Lu Q. Metabolomics in bladder cancer: a systematic review. Int J Clin Exp Med. 2015;8:11052–63. [PMC free article] [PubMed] [Google Scholar]
- 134. Ndaru E, Garibsingh RA, Shi Y, Wallace E, Zakrepine P, Wang J, Schlessinger A, Grewer C. Novel alanine serine cysteine transporter 2 (ASCT2) inhibitors based on sulfonamide and sulfonic acid ester scaffolds. J Gen Physiol. 2019;151:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mikkilineni L, Whitaker-Menezes D, Domingo-Vidal M, Sprandio J, Avena P, Cotzia P, Dulau-Florea A, Gong J, Uppal G, Zhan T et al.. Hodgkin lymphoma: A complex metabolic ecosystem with glycolytic reprogramming of the tumor microenvironment. Semin Oncol. 2017;44:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cruz-Bermúdez A, Laza-Briviesca R, Vicente-Blanco RJ, García-Grande A, Coronado MJ, Laine-Menéndez S, Palacios-Zambrano S, Moreno-Villa MR, Ruiz-Valdepeñas AM, Lendinez C et al.. Cisplatin resistance involves a metabolic reprogramming through ROS and PGC-1alpha in NSCLC which can be overcome by OXPHOS inhibition. Free Radic Biol Med. 2019;135:167–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.