Abstract
Background
The global COVID-19 pandemic has prompted an urgent search for interventions to prevent and treat SARS-CoV-2. Higher risk of infection and adverse outcomes coincide with populations with chronic diseases and elderly who are at risk of zinc deficiency. Through several mechanisms zinc may prevent, reduce severity and duration of symptoms.
Method
An a priori protocol was registered with PROSPERO on 27th April 2020 (CRD42020182044). Eight databases (one Chinese) and four clinical trial registries (one Chinese) were searched for randomised and quasi-randomised controlled trials (RCTs), evaluating single or adjunct zinc against placebo or active controls, for prevention and/or treatment of SARS-CoV-2, other coronaviruses or related infections. RR constraints included not searching bibliographies or contacting authors, single reviewers with calibration and second reviewer checking, meta-analyses and quality appraisal of critical and study primary outcomes only and reporting results as they became available.
Results
118 publications of 1,627 records met the inclusion criteria (35 Chinese and 83 English publications), 32 for prevention, 78 for treatment and 8 for both. Four RCTs specific to SARS-CoV-2 are ongoing; two are investigating zinc for prevention and two for treatment. As of 7 July 2020, no results were available. A wide range of zinc forms, including nasal spray/gel, lozenges, liquid, tablets and intramuscular were investigated.
Conclusion
Currently, indirect evidence suggests zinc may potentially reduce the risk, duration and severity of SARS-CoV-2 infections, particularly for populations at risk of zinc deficiency including people with chronic disease co-morbidities and older adults. Direct evidence to determine if zinc is effective for either prevention or treatment of SARS-CoV-2 is pending. In the interim, assessing zinc status of people with chronic diseases and older adults, as part of a SARS-CoV-2 clinical work-up, is reasonable as both groups have a higher risk of zinc deficiency/insufficiency and poorer outcomes from SARS-CoV-2.
Keywords: Zinc, Coronavirus, SARS-CoV-2, Rapid review
1. Brief overview
As of 9 June 2020, indirect evidence from other types of viral respiratory infections suggests that zinc may potentially reduce the risk, duration and severity of SARS-CoV-2 infections; particularly for populations at risk of zinc deficiency. Notably, people with chronic disease co-morbidities and older adults are at risk of lower zinc status. Pending the results of SARS-CoV-2 clinical trials, clinicians might consider assessing zinc status as part of a SARS-CoV-2 clinical work-up to determine if short-term zinc supplementation, either orally or intravenously is indicated for those with low or borderline low results, low dietary intake and/or increased needs.
2. Verdict
Zinc may potentially reduce the risk of SARS-CoV-2 infections and shorten the duration and severity of illness, including recovery from stroke, through several mechanisms. Indirect evidence from systematic reviews have found zinc supplementation is effective for the prevention of acute respiratory infections in young children and zinc lozenges may reduce the duration of the common cold in adults. Safety concerns associated with high doses or prolonged intake of zinc include anosmia (loss of smell) and copper deficiency.
As of the 9 June 2020, the preliminary findings of a rapid review of zinc for the prevention or treatment of SARS-CoV-2 and other viral respiratory tract infections included 122 randomised controlled trials (87 were published in English and 35 in Chinese). Only four were specific to SARS-CoV-2, and all are ongoing. Other ongoing SARS-CoV-2 trials are investigating the potential role of zinc as an agonist (additive) to hydroxychloroquine against placebo controls, or in combination with other nutraceuticals, most commonly Vitamin C and D. No other direct evidence pertaining to SARS-CoV-2 nor other coronavirus infections was identified. A detailed analysis of the indirect evidence, including meta-analyses, is underway.
Pending any definitive evidence, clinicians might consider assessing the zinc status of people with chronic disease co-morbidities and older adults as part of a SARS-CoV-2 clinical work-up, as both groups have a higher risk of zinc deficiency/insufficiency and poorer outcomes from SARS-CoV-2. Supplementation might be indicated for those with low or borderline low results, low dietary intake and/or increased needs.
3. Background
The global COVID-19 pandemic has prompted an urgent search for pharmaceutical and traditional, complementary and integrative medicine (TCIM) interventions. Data from all countries indicate that the case fatality and morbidity rates from SARS-CoV-2 increases with age and for those with non-communicable chronic disease co-morbidities. [1], [2], [3], [4] Notably, zinc deficiency/insufficiency is prevalent in populations aged over 71 years [5], [6], [7], [8], [9], in people with chronic diseases, [10], [11], [12] including diabetes [10], [12], [13], and cardiovascular diseases, [10], [12] and hospitalised patients following stroke [14] – see Box 1 .
Box 1. Populations at risk of zinc deficiency.
Insufficient dietary intake of zinc
Limited access to animal foods [15], [16], [17]
Consume plant based diets high in cereals, starchy roots, tubers and legumes (containing phytic acid which may reduce zinc bioavailability [17], [18]
Infants weaned from breast milk
Hospitalisation following stoke [14]
People with increased biological need
Pregnant and breastfeeding women [19], [20]
Early post-natal infants [20], [21]
Children [19]
Chronic diseases (e.g. chronic obstructive pulmonary diseases, asthma, anaemia, renal disease, inflammatory bowel and other chronic gastrointestinal diseases, HIV, Alzheimer’s disease, rheumatoid arthritis) [22], [23]
Alcohol abuse [24]
Insufficient dietary intake of zinc
Limited access to animal foods [15], [16], [17]
Consume plant based diets high in cereals, starchy roots, tubers and legumes (containing phytic acid which may reduce zinc bioavailability [17], [18]
Infants weaned from breast milk
Hospitalisation following stoke [14]
People with increased biological need
Pregnant and breastfeeding women [19], [20]
Early post-natal infants [20], [21]
Children [19]
Chronic diseases (e.g. chronic obstructive pulmonary diseases, asthma, anaemia, renal disease, inflammatory bowel and other chronic gastrointestinal diseases, HIV, Alzheimer’s disease, rheumatoid arthritis) [22], [23]
Alcohol abuse [24]
Alt-text: Box 1
Zinc is widely available for self-prescribed use and is a common naturopathic medicine used for a variety of clinical indications, including the prevention and treatment of viral respiratory infections, tissue repair and supporting a healthy immune system [25]. Zinc plays an important role in immune function, wound healing, insulin and blood pressure regulation, and the regulation of gene expression [26]. Zinc may be formulated as a stand-alone nutraceutical or as a combination product containing other minerals, vitamins and/or herbs. Most zinc supplements are administered orally either in single or divided daily doses, in the form of a lozenge, tablet, capsule, liquid or syrup. Some products are formulated for intramuscular or intravenous administration.
Zinc supplementation is not without potential safety concerns, that includes anosmia [27] and copper deficiency associated with higher doses and prolonged intake [28]. The daily recommended dietary intake (RDI) of elemental zinc is around 2 mg for infants up to 6 months of age, and gradually increases to 11 mg for males, and 8 mg per day for females older than 13 years [29]. Tolerable upper limits for zinc are estimated to be 7 mg for children aged 1–3 years of age, increasing up to 25 mg for adults and females of any age who are pregnant or lactating. The no observed adverse effect level (NOAEL) for adults is around 50 mg/day [28].
Over 17 % of the global population is estimated to be zinc deficient [30], and 20 % of national diets contain insufficient zinc to meet minimum health requirements [31], [32]. Deficiency is highest in South-East Asia, Sub-Saharan and Central and South American regions, however, marginal deficiencies are also prevalent in developed regions [33], [34].
Assessment of zinc status is notoriously difficult due to absence of sensitive and precise biochemical indicators. The most reliable methods involve combining a clinical assessment with laboratory tests assessing tissue concentrations of zinc in plasma or hair [35]. Clinical manifestations of mild-moderate zinc deficiency include recurrent infections, slow tissue repair, rough skin, mental lethargy, irritability, headaches and reduced lean body mass [36]. Assessment of dietary zinc with validated food frequency instruments may help identify dietary insufficiency [37] however zinc status is still likely to be underestimated due to individual physiological characteristics [31]. For instance, whilst zinc insufficiency/deficiency is known to diminish antibody and cell-mediated immunity in humans that in turn increases the risk of infections, this may only become apparent upon immune system provocation [38], [39].
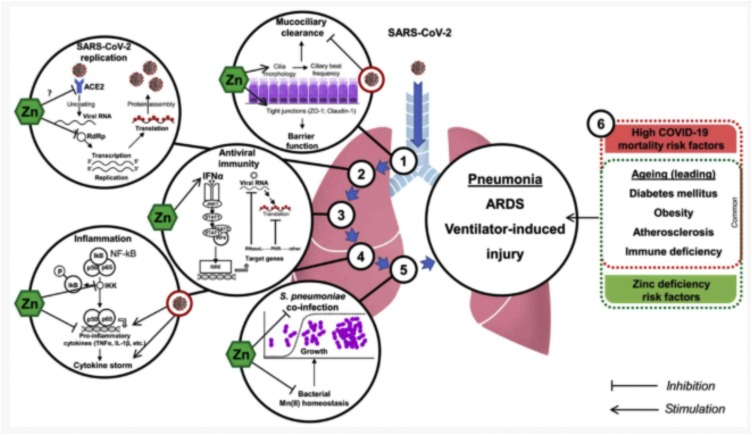
Through several mechanisms, zinc has the potential to reduce the risk of viral respiratory tract infections, including SARS-CoV-2, and shorten the duration and severity of illness. The authors of a recent non-systematic narrative review of the underlying mechanisms postulate that along with its direct antiviral properties, zinc has the potential to reduce inflammation, improve mucocillary clearance, prevent of ventilator-induced lung injury, and modulate antiviral immunity [40] (Fig. 1 ).
Fig. 1.
The proposed protective mechanisms of zinc in Covid-19. From “Zinc and respiratory tract infections: Perspectives for COVID‑19 (Review)” by Skalny, A.V., Rink, L., Ajsuvakova O. et al. 2020 in the International Journal of Molecular Medicine; Volume 46, Issue 1, page 21. Copyright Spandidos Publications [41].
In vitro studies have demonstrated that zinc can inhibit the enzymatic activity and replication of SARS-CoV RNA polymerase and may inhibit angiotensin‑converting enzyme 2 (ACE2) activity [40], [42], [43]. The antiviral effects of zinc are also hypothesised to potentiate the therapeutic effects of chloroquine [44], as chloroquine acts as a zinc ionophore increasing Zn2+ influx into the cell [40]. Zinc may also modify the host’s response to an infection as it is an essential co-factor element with a broad range of functions in the body. Zinc has an essential role in immune and airways function, wound healing and tissue repair that in turn, may delay or prevent recovery from viral respiratory illnesses [45], [46], [47], [48], [49], [50], [51]. Other consequences of zinc deficiency include an increased risk of vitamin A deficiency that is also critical for immune function, due to carrier proteins and activation enzymes being dependant on sufficient zinc status [52].
The potential role of zinc as an adjuvant therapy for SARS-CoV-2 may be broader than just antiviral and/or immunological support. Zinc also plays a complex role in haemostatic modulation acting as an effector of coagulation, anticoagulation and fibrinolysis [53], [54]. Zinc is also essential for neurological function and normalisation of zinc intake has been shown to improve neurological recovery following stroke [14].
The effectiveness of zinc in preventing or treating SARS-CoV-2 infections is yet to be systematically evaluated and, along with other nutritional supplements, was not mentioned in a recent narrative review of TCIM for the treatment of coronavirus disease 2019 (COVID-19) [55]. The findings of systematic reviews of related populations are promising; however, the reviews are limited by population, intervention, or are out of date [56], [57], [58].
A 2016 Cochrane review of six RCTs concluded zinc supplementation was effective for the prevention of pneumonia in children aged two to 59 months [57]. Unlike an earlier review in 2000 of seven RCTs with adult participants and one RCT with children [59], an updated 2011 systematic review of 13 RCTs found a dose-dependent effect of zinc lozenges compared to placebo controls for reduced duration of common colds in adults [60]. Daily dosages less than 75 mg of zinc had no significant effect on duration of colds, however, daily dosage over 75 mg reduced the duration of colds by 42 % (95 % CI: 35 %–48 %). In a subsequent 2017 systematic review of seven RCTs of zinc lozenges with a daily dose >75 mg, a smaller reduction of 33 % (95 % CI 21 %–45 %) in the duration of common colds was found [61]. No differences in duration were found for daily doses of 192−207 mg compared to doses of 80−92 mg.
Other formats of zinc for preventing or treating upper respiratory infections were examined in three Cochrane systematic reviews, however, all were withdrawn [56], [62], [63]. A protocol for the systematic review of zinc for prevention and treatment of common colds was withdrawn in 2019 due to non-completion within the editorial time-frame [64].
4. Search strategy
4.1. Research questions
The primary objective of this rapid review was to assess the effects of zinc on the incidence, duration and severity of acute upper or lower respiratory tract infections caused by SARS-CoV-2 infection in people of any age and of any zinc status when used as a preventive supplement or as a therapy.
The secondary objectives are to assess the effects of zinc on the incidence, duration and severity of acute upper or lower respiratory tract infections
-
1
caused by other coronavirus species, with a focus on SARS-CoV and MERS-CoV infections;
-
2
predominantly caused by viruses; and
-
3
in subgroups of populations at risk of zinc insufficiency/deficiency and those with a higher risk of severe acute respiratory syndrome (SARS) caused by SARS-CoV-2 infection.
4.2. Protocol
A protocol for this rapid review outlining the methods in detail, including the methodological constraints employed to facilitate a timely answer to the review questions, was registered on 24 April 2020 with PROSPERO: CRD42020182044 [65].
Rapid review method constraints included not systematically searching the bibliographies of included articles, and jointly screening (SA, JH, GY, JG), only 30 title/abstracts and 5 full-text articles for calibration and consistency, after which only one reviewer (SA, JH, GY, JG) screened each article. Similarly, only three studies and their outcomes were jointly assessed (SA, GY JH) for calibration and consistency using the Cochrane RoB 2.0 tool [66], [67] and a piloted rubric. Study characteristics and data were extracted into a piloted electronic spreadsheet, after which only one reviewer (SA, GY, JH) assessed RoB and extracted data for each study.
4.3. Inclusion/exclusion criteria
4.3.1. Included
Primary studies included were randomized controlled trials (RCTs) and quasi-randomised controlled trials. There were no date nor language restrictions, however, studies published in languages other than English or Chinese are yet to be translated.
Included were people of any age, gender and zinc status in any setting who are 1) at risk of contracting an acute upper or lower viral respiratory tract infection, including healthy populations, 2) have a confirmed SARS-CoV-2 or other respiratory infection caused by a coronavirus species, including SARS-CoV and MERS-CoV, and/or 3) have either a laboratory confirmed viral respiratory tract infection (any virus) or an acute respiratory tract infection where the cause is most likely viral such as the common cold, non-seasonal rhino-sinusitis, laryngitis, flu-like illness, healthy people with acute bronchitis, or young children with pneumonia.
Included were any zinc conjugates, such as salts or amino-chelates as a single ingredient, in any form (e.g. tablet, syrup, lozenge, gel, spray, liquid), dose and duration, administered via oral, intranasal, sublingual, transdermal, intramuscular or intravenous routes.
4.3.2. Excluded
Excluded were systematic reviews, non-randomised studies of interventions and studies without a concurrent control, such as case series and case reports.
Excluded were people with respiratory tract infections or other upper/lower respiratory illnesses when the cause was confirmed not to be a viral infection, or a non-viral cause is common.
Excluded were co-interventions and zinc administered alongside other nutraceuticals, herbs or pharmaceuticals unless both the intervention and control groups received the co-intervention. The exception were co-ingredients with the primary purpose to facilitate absorption (e.g. vitamin B12) or cellular retention (e.g. vitamin B6 or magnesium) of zinc.
4.4. Databases
The following databases were searched from inception: PubMed on the 8 May 2020, selected EBSCO host databases (Academic Search Complete, Allied and Complementary Medicine Database (AMED), Alt Health Watch, CINAHL Plus with Full Text, Health Source, and PsycINFO), Embase and Cochrane CENTRAL on the 27 April 2020, and the China Knowledge Resource Integrated Database (CNKI) on 29 April 2020.
Additionally, the following clinical trials registries were searched for SARS-CoV-2 infections only. The U.S. National Library of Medicine Register of Clinical Trials (ClinicalTrials.gov), International Standard Randomized Controlled Trial Number Register (ISRCTN) and World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) were searched on 5 May 2020. The Chinese Clinical Trial Registry was searched on 29 April 2020.
4.5. Search terms (example)
4.5.1. PubMed - Boolean/phrase
(Coronaviridae[mh] OR Coronavir* OR nCov OR covid OR Coronaviridae Infections[mh] OR Middle East Respiratory Syndrome Coronavirus[mh] OR "Middle East Respiratory Syndrome" OR MERS OR "Severe Acute Respiratory Syndrome" OR “Severe acute respiratory syndrome-related coronavirus” OR “Severe Acute Respiratory failure” OR “Acute febrile respiratory syndrome” OR SARS OR Respiratory Tract Infections[mh] OR “Lower respiratory infection” OR “viral respiratory” OR pneumonia OR “flu -like illness” OR bronchitis OR “Common cold” OR Rhinitis OR laryngitis OR “Respiratory Infections” OR “Infections, respiratory” OR “Infections, Respiratory Tract” OR “Infections, Upper Respiratory” OR “Upper Respiratory Tract” OR “Infections, Lower Respiratory Infections” OR “Lower Respiratory Infections” OR “Lung Inflammation” OR “Lobar Pneumonia” OR “Lobar Pneumonitis” OR “Pulmonary Inflammation”) AND (Zinc[mh] OR zinc OR zn) AND (randomized controlled trial[pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])
5. Results
A total of 1625 records were retrieved from the database searches, of which 1182 records remained after duplicates were removed. A further 981 records were excluded at title and abstract screening, and 80 following full-text screening (due to ineligible study design n = 29, population n = 11 or intervention n = 32; full-text not available n = 7; or awaiting translation n = 1), leaving 121 records reporting 122 primary studies (86 published in English and 35 in Chinese). One study published in Spanish is pending translation.
Four trials specific to SARS-CoV-2 were included, all of which are currently ongoing, and the investigators have been contacted are yet to report their results. A further 15 ongoing trials were excluded as the interventions used zinc in combination with other nutraceuticals (most commonly vitamin C and D) and/or as an agonist (additive) to hydroxychloroquine. As such, the independent effects of zinc cannot be determined.
Of the remaining 118 published studies, none investigated zinc for prevention or treatment of acute respiratory infections caused only by a coronavirus infection. Most of the studies (79 %) evaluated zinc for treating or preventing upper and/or lower acute respiratory infections in children. (Table 1 ). All the studies of adult participants were for acute upper respiratory infections i.e. the common cold (Table 1), of which 21 were naturally occurring infections and six inoculated the participants with human rhinovirus species.
Table 1.
RCTs and quasi-RCTs of zinc for acute viral respiratory infections.
| STUDY AIM | Adults | Children | TOTAL |
|---|---|---|---|
| Database source | |||
| Zinc for treatment only | 19 | 59 | 78 |
| CKNI | 0 | 31 | 31 |
| Other databases | 19 | 28 | 47 |
| Zinc for prevention only | 3 | 29 | 32 |
| CKNI | 2 | 1 | 3 |
| Other databases | 1 | 28 | 29 |
| Zinc for prevention and treatment | 3 | 5 | 8 |
| CKNI | 0 | 1 | 1 |
| Other databases | 3 | 4 | 7 |
| TOTAL | 25 | 93 | 118 |
CNKI: China Knowledge Resource Integrated Database.
The prevention effect of zinc was assessed in a variety of ways, mostly as the incidence or recurrence of respiratory infections as reported by study clinicians, participants’ physician or healthcare providers, parents or self-reports, hospitalisation and/or laboratory tests. Treatment effects for severity and duration included time to symptom resolution, fever or respiratory distress, time in hospital, viral shedding, and self or clinician reported clinical severity.
A wide range of zinc formulations and dosages were used, including lozenges, nasal gels and sprays, and oral zinc delivered in syrup, tablet or capsule formats. Only one study evaluated intravenous zinc.
Most studies were conducted in community settings. The studies of children were representative of all WHO regions, whereas most of the adult studies were conducted in the United States (n = 19), three were in the WHO Western Pacific Region and in WHO Europe Region.
6. Summary of findings
Currently, there is no direct evidence to determine if zinc is effective for either the prevention or treatment of SARS-CoV2-19. The protocols of four RCTs have been registered and are currently recruiting, which are summarised in Table 2 . One aims to evaluate zinc on the clinical course of SARS-CoV2-19 in non-hospitalised participants in the community. The second will compare zinc against placebo controls for oxygen saturation in hospitalised patients admitted with SARS-CoV2-19. The third and fourth registered trials aim to compare the effectiveness of zinc as adjunct treatment to hydroxychloroquine, one study for prevention and the other for treatment of SARS-CoV-2. The third and fourth trials include participants from populations at high risk of zinc deficiency.
Table 2.
Registered randomized control trials of zinc for SARS-CoV-2 (9 June 2020).
| HCQ and Zinc in the Prevention of COVID-19 Infection in Military Healthcare Workers (COVID-Milit) | |
|---|---|
| Registration no. | NCT04377646 |
| Registration date | 4 May 2020 |
| Completion date | 31 July 2020 (not confirmed) |
| Location | Tunisia |
| Setting | Tunisia Military Academy |
| Design | Multicentre, double-blind RCT, 3 arms |
| Sample size | N = 660 |
| Demographics | Military professionals aged 18–65 |
| Inclusion criteria | At risk of infection by SARS-CoV-2 at 2 levels |
| Exclusion criteria | 1. Allergy to medications |
| 2. Heart rhythm disturbances | |
| 3. Severe hepatic impairment | |
| 4. Retinal pathology | |
| 5. Epilepsy | |
| 6. Myasthenia | |
| 7. Psoriasis | |
| 8. Methemoglobinemia | |
| 9. Porphyria | |
| 10. Pregnant or lactating women | |
| 11. Concomitant treatments | |
| Zinc intervention (elemental dose) | Zinc capsules 15 mg/day + HCQ 400 mg on day 1 and 2 and HCQ 400 mg/week for 2 months |
| Comparator | 1. Placebo zinc, 1 per day for 28 days + HCQ 400mg on day 1 and 2 and 400 mg/week for 2 months |
| 2. Placebo zinc, 1 each day + placebo HCQ on day 1 and 2 and weekly for 2 months | |
| Primary Outcomes | Incidence of SARS CoV2 infection |
| Secondary Outcomes | 1. Incidence of any COVID-19 related symptoms |
| 2. Adverse events | |
| Follow-up time | 28 days |
| Coronavirus 2019 (COVID-19) - Using Ascorbic Acid and Zinc Supplementation (COVIDAtoZ) | |
|---|---|
| Registration no. | NCT04342728 |
| Registration date | 8 April 2020 |
| Completion date | 8 April 2020 |
| Location | US |
| Setting | Community health clinics and hospital outpatients, Ohio and Florida |
| Design | Multicentre, open label RCT, 4 arms |
| Sample size | N = 520 |
| Demographics | Adults, including women of child-bearing potential |
| Inclusion criteria | Confirmed diagnosis of SARS-CoV-2 not requiring hospitalisation |
| Exclusion criteria | 1. SARS-CoV-2 detected during hospitalisation |
| 2. Pregnant and lactating | |
| 3. CKD | |
| 4. Liver disease (waiting transplant) | |
| 5. Calcium oxalate stones | |
| Zinc intervention (elemental dose) | 1. Zinc gluconate 50 mg (7 mg)/day for 28 days |
| 2. Zinc gluconate 50mg (7 mg)/day + vitamin C 8000 mg/day for 28 days | |
| Comparator | 1. Usual (standard) care |
| 2. Vitamin C alone | |
| Primary Outcomes | Days to 50% reduction of symptoms |
| Secondary Outcomes | 1. Symptom resolution |
| 2. Total symptom score on day 5 | |
| 3. Hospitalisation | |
| 4. Adjunctive medicines | |
| 5. Adverse events | |
| Follow-up time | 28 days |
| The effect of zinc on the treatment and clinical course of patients with SARS-cov2 (COVID-19) | |
|---|---|
| Registration no. | IRCT20180425039414N2 |
| Registration date | 31 May 2020 |
| Completion date | NI |
| Location | Iran |
| Setting | Amin Hospital, Isfahan |
| Design | Open label RCT, 2 arms |
| Sample size | N = 80 |
| Demographics | Adults |
| Inclusion criteria | Hospitalised with confirmed SARS-CoV-2 infection (RT, PCR and CT scan of the lungs). Blood oxygen levels: 90–3%; Breathing rate 20–24 breaths/min; Heart rate 100–130 bpm |
| Exclusion criteria | 1. Intubation |
| 2. Blood oxygen below 90% Breathing rate equal to 30 or more breaths per minute | |
| 3. Allergic to interventions | |
| 4. Cardiogenic pulmonary oedema associated shortness of breath | |
| 5. Pregnancy and lactation | |
| 6. Oxygen therapy at home | |
| 7. End stage lung, malignant, G6PD deficiency, diabetic ketoacidosis, cardiac arrhythmia | |
| Zinc intervention (elemental dose) | Zinc tablets 440 mg/day + HCQ sulphate tablets 400mg every 12 hours on day 1 and 200 mg every 12 hours during hospitalisation |
| Comparator | HCQ sulphate tablets 400mg every 12 hours on day 1 and 200mg every 12 hours during hospitalisation |
| Primary Outcomes | Clinical course defined as: |
| 1. Resolution of symptoms (fever, shortness of breath, cough), SaO2 and hemodynamic parameters | |
| 2. Mortality | |
| 3. Days in hospital | |
| Secondary Outcomes | None |
| Follow-up time | During hospitalisation |
| High-dose intravenous zinc (HDIVZn) as adjunctive therapy in COVID-19 positive critically ill patients: A pilot randomized controlled trial | |
|---|---|
| Registration no. | ACTRN12620000454976 |
| Registration date | 8 April 2020 |
| Completion date | |
| Location | Australia |
| Setting | Austin Hospital, Victoria |
| Design | Pilot RCT |
| Sample size | N=160 |
| Demographics | Adults |
| Inclusion criteria | Hospitalised with confirmed SARS-CoV-2 infection (PCR or other laboratory confirmed) of any duration. SaO2: ≤94% or Pao2:Fio2 ≤300 mg Hg. Ventilated or non-ventilated. |
| Exclusion criteria | 1.CKD |
| 2. Pregnant or lactating | |
| 3. Allergy to Zinc | |
| 4. Severe hepatic impairment | |
| 5. eGFR ≤30 mL/min/1.73 m2 | |
| 6. Organ transplant | |
| 7. CPR within 14 days | |
| 8. DNR or DNI orders | |
| 9. Imminent or inevitable death | |
| 10. Dialysis | |
| 11. HIV infection | |
| 12. Known or suspected history of oxalate nephropathy or hyperoxaluria, scurvy, chronic iron overload, G-6PD deficiency | |
| Zinc intervention (elemental dose) | Zinc 0.5 mg/kg/day intravenous infusion (saline 250 ml/day) over 3–6 hrs for 7 days |
| Comparator | Saline solution 250 ml/day infused over 3–6 hrs for 7 days |
| Primary Outcomes | For non-ventilated patients: mean change in the worst (highest) level of oxygenation (flow in litres/min) For ventilated patients: mean change in the worst (lowest) PaO2:FiO2(mmHg) Feasibility: blinding; drug availability; GCP; protocol compliance; costs; SOP |
| Secondary Outcomes | 1. Mortality |
| 2. Duration of mechanical ventilation | |
| 3. Duration of oxygen therapy | |
| Follow-up time | 28 days |
6GPD, Glucose-6-phosphate dehydrogenase deficiency; CPR, cardiopulmonary resuscitation; CT, computerized tomography; DNR, do not resuscitate; DNI, do not intubate; eGFR, estimated Glomerular Filtration Rate; GCP, Good Clinical Practice FiO2 fraction of inspired oxygen; HCQ, hydroxychloroquine: HCQ, hydroxychloroquine; PaO2, Partial pressure of oxygen; PCR, polymerase Chain Reaction; RCT, randomised controlled trial; RT, Rapid Test; SaO2, Oxygen saturation; SOP, standard operating procedures.
The first study, “Coronavirus 2019 (COVID-19) - Using Ascorbic Acid and Zinc Supplementation (COVIDAtoZ)” (NCT04342728), plans to recruit 520 adults with a confirmed SARS-CoV-2 infection who do not require hospital admission. Based in a community setting in the United States (US), COVIDAtoZ is a four-arm pragmatic RCT comparing zinc gluconate only, zinc gluconate and Vitamin C, Vitamin C only, and usual care (standard prescribed medication/supplements). The dose of zinc gluconate is 50 mg daily, taken at bedtime. The primary outcome is the number of days required to reach a 50 % reduction in symptom severity score (derived from a composite self-rating score of fever, cough, shortness of breath and fatigue rated on a 0–3 scale). Secondary outcomes are time to symptom resolution for each symptom, total symptom composite score at day 5, proportion requiring hospitalisation, use of prescribed adjunctive medicines, and adverse events. Methodological limitations include subjective primary outcome measures from unblinded participants, potential uncertainty around the quality and quantity of the ingredients in the supplements [68], a potentially insufficient dose of elemental zinc and that the usual care group may use any combination of readily available prescribed medications / supplements, including zinc or vitamin C. Strengths of the pragmatic design include a capacity to inform ‘real-world’ decisions about any benefits and risks of additional zinc supplementation using products that are readily available compared to usual care alone.
The second study, “High-dose intravenous zinc (HDIVZn) as adjunctive therapy in COVID-19 positive critically ill patients: A pilot randomized controlled trial” (ACTRN12620000454976), is being conducted in a hospital setting in Australia. HDIVZn is a two-arm, double-blind RCT comparing intravenous zinc chloride (0.5 mg/kg/d) or placebo in 250 ml saline bags infused daily over 3−6 h for seven days. HDIVZn aims to recruit 160 patients who are hospitalised with SARS-CoV-2 infection. The primary outcome is oxygenation. Secondary outcomes are concerned with feasibility, including adequacy of blinding, availability/delivery/storage of the zinc infusions and per-patient costs. Methodological strengths include blinding and the use of an objective primary outcome measure. Limitations include not assessing any other clinical outcomes listed in the core outcome set (COS) for clinical trials on COVID-19 [4]. The dose of zinc, approximately 50 % more than the minimum daily requirement and without an intracellular transporter co-factor, may be insufficient to effect change of the outcome measurements [69]. Given this is a single-centre trial located in Australia with a low incidence of SARS-CoV-2, as of the 14th June 2020, no eligible participants had been recruited to the study; and, according to the investigator A/Professor Ischia, “due to the low numbers of COVID-19 infections, the trial is unlikely to reach full recruitment to achieve its desired statistical power” [70].
Prevention of SARS-CoV-2 is being evaluated in a multicentre trial of 660 military health professionals exposed to SARS-CoV-2 and located in Tunisia (NCT04377646: A Study of Hydroxychloroquine and Zinc in the Prevention of COVID-19 Infection in Military Healthcare Workers (COVID-Milit)). Participants will be randomized to one of three study arms; either hydroxychloroquine and zinc, hydroxychloroquine and placebo, or two placebo controls. In COVID-Milit, hydroxychloroquine 400 mg will be administered at day 1 and day 2, then as a weekly dose for up to 2 months. Zinc will consist of 15 mg per day for up to two months. The primary outcome is the frequency of infection at two months, secondary outcomes are frequency of ten symptoms and adverse events. The low dose of zinc will provide minimum intake required for health.
The treatment of SARS-CoV-2 with either hydroxychloroquine plus zinc compared to hydroxychloroquine alone will be evaluated in 80 hospitalised adults with confirmed SARS-CoV-2. This study, registered on the Iranian clinical registry (IRCT20180425039414N2; The effect of zinc on the treatment and clinical course of patients with SARS-CoV2 (COVID-19)), is being conducted at the Amin Hospital in Isfahan. Participants will be randomised to either zinc 220 mg twice daily plus Hydroxychloroquine 20 mg every 12 h, or to hydroxychloroquine alone during their hospital stay. Outcomes include mortality rates, length of hospital stay and the clinical course of SARS-CoV-2 (fever, shortness of breath, cough, blood oxygenation (SaO2) and hemodynamic parameters). The treatment study was designed to ensure that all study participants diagnosed with SARS-CoV-2 received treatment.
7. Clinical significance
Preliminary findings of this rapid systematic review found limited direct evidence evaluating zinc for the prevention or treatment of SARS-CoV-2, as results of four registered RCTs are pending. Once available, the findings from the COVIDAtoZ trial that is evaluating the comparative effectiveness of zinc supplements against vitamin C and usual care for treatment of mild to moderate symptoms of community-based SARS-CoV2-19, will be relevant to the general population who can self-prescribe, along with a wide range of health practitioners who provide TCIM advice. The findings from the HDIVZn trial evaluating the efficacy and safety of intravenous zinc infusions for hospitalised patients, may provide safer and less expensive therapeutic options compared to other pharmaceuticals currently being evaluated. Delivery of the intervention, however, requires medical oversight that will restrict its application to hospital settings and perhaps a few primary care settings. The two comparative effectiveness studies will not explain the preventative or treatment effects of zinc as a stand-alone therapy, however they will explain the potential benefits of zinc adjunct to hydroxychloroquine in populations at high risk of zinc deficiency [34], for prevention of SARS-CoV-2 in health professionals and for treatment of patients hospitalised due to SARS-CoV-2.
In contrast, a substantial volume of indirect clinical evidence from RCTs investigating zinc for preventing and/or treating acute respiratory infections commonly caused by viruses was identified. Only 20 of the 120 RCTs included in this rapid review have previously been meta-analysed and whilst the results are promising they are limited to infants (n = 6) [57], children (n = 1) [59] and zinc lozenges in adults (n = 13) [59], [60], [61]. The studies identified in this rapid review therefore warrant further in-depth appraisal and meta-analysis where possible. To facilitate the rapid dissemination of results that are most relevant to populations at a higher risk of morbidity and mortality from SARS-CoV-2, an analysis of the 20 RCTs of zinc for upper respiratory tract infections in adults will be undertaken first prior to analysing the studies involving children. Whilst the grading of the evidence will be downrated due indirectness, in the absence of more direct evidence, the findings are clinically relevant as an estimated 15 % of upper respiratory tract infections in adults are caused by coronaviruses [71].
Along with the positive findings from the limited systematic reviews to date, the rationale for the use of zinc in SARS-CoV-2 prevention and treatment, and possibly rehabilitation, is supported by the known mechanistic actions of zinc as an antiviral agent [40], [42], [43], and a key element for a broad range of functions in the body that modulate immunity, respiratory tract inflammation, coagulation and neurological function to name a few [14], [38], [39], [40], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54].
Pending any definitive evidence, it might be reasonable for clinicians to consider assessing the zinc status of people with chronic disease co-morbidities and older adults as part of a SARS-CoV-2 clinical work-up, as both groups have a higher risk of zinc deficiency/insufficiency and poorer outcomes from SARS-CoV-2. Zinc status can be assessed by taking a diet and clinical history (see Box 1), clinical examination and laboratory tests. Plasma zinc may be more reliable than serum zinc and whilst hair mineral analysis is another option although a timely result may not be available [35].
For prevention of SARS-CoV-2 and most importantly for general health, given that zinc supplements are readily available, they may be indicated for people with low or borderline low results, low dietary intake and/or increased needs. To optimise safety, a daily dose lower than the tolerable upper limits (<7 mg for children aged 1–3 years up to 22 mg for those aged 15–17 years) should be used along with dietary modifications whenever possible. In adults, doses up to the no observed adverse effect level (NOAEL) of 50 mg/day should be considered [28]. At this stage, it is unclear if there is any additional benefit from supplementing zinc for the prevention of SARS-CoV-2 or other viral respiratory infections in low risk populations nor for people with normal zinc status.
It is also unclear if there are any benefits from supplementing with zinc for the treatment of SARS-CoV-2. There is limited indirect evidence from viral upper respiratory infections that zinc lozenges with a daily dose of >75 mg of zinc may shorten the duration of the common cold. However, there are risks with higher doses above the NOAEL including permanent loss of smell [28]. Therefore, a daily dose higher than 100 mg of elemental zinc in a lozenge is probably not advisable, as it is questionable whether there are any additional therapeutic effects [61].
Disclaimer
This article should not replace individual clinical judgement. The views expressed in this rapid review are the views of the authors and not necessarily from the host institutions. The views are not a substitute for professional medical advice.
References
- 1.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls. StatPearls Publishing; 2020. Features, evaluation and treatment coronavirus (COVID-19) [Internet]. edn. [PubMed] [Google Scholar]
- 2.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., Qin R., Wang H., Shen Y., Du K. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab. Res. Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y.-H., Cai L., Cheng Z.-S., Cheng H., Deng T., Fan Y.-P., Fang C., Huang D., Huang L.-Q., Huang Q. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett J.B., Hamer D.H., Meydani S.N. Low zinc status: a new risk factor for pneumonia in the elderly? Nutr. Rev. 2010;68(1):30–37. doi: 10.1111/j.1753-4887.2009.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ervin R.B., Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J. Nutr. 2002;132(11):3422–3427. doi: 10.1093/jn/132.11.3422. [DOI] [PubMed] [Google Scholar]
- 7.Haase H., Mocchegiani E., Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology. 2006;7(5–6):421–428. doi: 10.1007/s10522-006-9057-3. [DOI] [PubMed] [Google Scholar]
- 8.Pepersack T., Rotsaert P., Benoit F., Willems D., Fuss M., Bourdoux P., Duchateau J. Prevalence of zinc deficiency and its clinical relevance among hospitalised elderly. Arch. Gerontol. Geriatr. 2001;33(3):243–253. doi: 10.1016/s0167-4943(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 9.Briefel R.R., Bialostosky K., Kennedy-Stephenson J., McDowell M.A., Ervin R.B., Wright J.D. Zinc intake of the US population: findings from the third National Health and Nutrition Examination Survey, 1988–1994. J. Nutr. 2000;130(5):1367S–1373S. doi: 10.1093/jn/130.5.1367S. [DOI] [PubMed] [Google Scholar]
- 10.Prasad A.S. Discovery of human zinc deficiency: its impact on human health and disease. Adv. Nutr. 2013;4(2):176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devirgiliis C., Zalewski P.D., Perozzi G., Murgia C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat. Res. Mol. Mech. Mutagen. 2007;622(1–2):84–93. doi: 10.1016/j.mrfmmm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Soinio M., Marniemi J., Laakso M., Pyörälä K., Lehto S., Rönnemaa T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care. 2007;30(3):523–528. doi: 10.2337/dc06-1682. [DOI] [PubMed] [Google Scholar]
- 13.Kazi T.G., Afridi H.I., Kazi N., Jamali M.K., Arain M.B., Jalbani N., Kandhro G.A. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol. Trace Elem. Res. 2008;122(1):1–18. doi: 10.1007/s12011-007-8062-y. [DOI] [PubMed] [Google Scholar]
- 14.Aquilani R., Baiardi P., Scocchi M., Iadarola P., Verri M., Sessarego P., Boschi F., Pasini E., Pastoris O., Viglio S. Normalization of zinc intake enhances neurological retrieval of patients suffering from ischemic strokes. Nutr. Neurosci. 2009;12(5):219–225. doi: 10.1179/147683009X423445. [DOI] [PubMed] [Google Scholar]
- 15.Allen L.H. The nutrition CRSP: what is marginal malnutrition, and does it affect human function? Nutr. Rev. 1993;51(9):255–267. doi: 10.1111/j.1753-4887.1993.tb03117.x. [DOI] [PubMed] [Google Scholar]
- 16.Ruel M.T., Bouis H.E. Plant breeding: a long-term strategy for the control of zinc deficiency in vulnerable populations. Am. J. Clin. Nutr. 1998;68(2):488S–494S. doi: 10.1093/ajcn/68.2.488S. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson E.L., Gibson R.S., Thompson L.U., Ounpuu S. Dietary calcium, phytate, and zinc intakes and the calcium, phytate, and zinc molar ratios of the diets of a selected group of East African children. Am. J. Clin. Nutr. 1989;50(6):1450–1456. doi: 10.1093/ajcn/50.6.1450. [DOI] [PubMed] [Google Scholar]
- 18.Foster M., Chu A., Petocz P., Samman S. Effect of vegetarian diets on zinc status: a systematic review and meta‐analysis of studies in humans. J. Sci. Food Agric. 2013;93(10):2362–2371. doi: 10.1002/jsfa.6179. [DOI] [PubMed] [Google Scholar]
- 19.Shah D., Sachdev H.S. Effect of gestational zinc deficiency on pregnancy outcomes: summary of observation studies and zinc supplementation trials. Br. J. Nutr. 2001;85(S2):S101–S108. doi: 10.1079/bjn2000301. [DOI] [PubMed] [Google Scholar]
- 20.Hess S., King J. Effects of maternal zinc supplementation on pregnancy and lactation outcomes. Food Nutr. Bull. 2009;30(1 Suppl):S60–78. doi: 10.1177/15648265090301S105. [DOI] [PubMed] [Google Scholar]
- 21.Karimi A., Bagheri S., Nematy M., Saeidi M. Zinc deficiency in pregnancy and fetal-neonatal outcomes and impact of the supplements on pregnancy outcomes. Iran. J. Neonatol. IJN. 2012;3(2):77–83. [Google Scholar]
- 22.Fraker P.J., King L.E., Laakko T., Vollmer T.L. The dynamic link between the integrity of the immune system and zinc status. J. Nutr. 2000;130(5):1399S–1406S. doi: 10.1093/jn/130.5.1399S. [DOI] [PubMed] [Google Scholar]
- 23.Tudor R., Zalewski P., Ratnaike R. Zinc in health and chronic disease. J. Nutr. Health Aging. 2005;9(1):45–51. [PubMed] [Google Scholar]
- 24.Mehta A.J., Yeligar S.M., Elon L., Brown L.A., Guidot D.M. Alcoholism causes alveolar macrophage zinc deficiency and immune dysfunction. Am. J. Respir. Crit. Care Med. 2013;188(6):716–723. doi: 10.1164/rccm.201301-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boon H.S., Cherkin D.C., Erro J., Sherman K.J., Milliman B., Booker J., Cramer E.H., Smith M.J., Deyo R.A., Eisenberg D.M. Practice patterns of naturopathic physicians: results from a random survey of licensed practitioners in two US States. BMC Complement. Altern. Med. 2004;4(1):14. doi: 10.1186/1472-6882-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chasapis C.T., Ntoupa P.-S.A., Spiliopoulou C.A., Stefanidou M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020:1–18. doi: 10.1007/s00204-020-02702-9. [DOI] [PubMed] [Google Scholar]
- 27.Eby G.A., Halcomb W.W. Ineffectiveness of zinc gluconate nasal spray and zinc orotate lozenges in common-cold treatment: a double-blind, placebo-controlled clinical trial. Altern. Ther. Health Med. 2006;12(1):34–38. [PubMed] [Google Scholar]
- 28.European Commission Health & Consumer Protection Directorate-General: Scientific Committee on Food; 2003. Opinion of the Scientific Committee on Food on the Tolerable upper Intake Level of Zinc (expressed on 5 March 2003) SCF/CS/NUT/UPPLEV/62 Final. [Google Scholar]
- 29.National Academies Press (US); Washington (DC): 2001. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Institute of Medicine Panel on Micronutrients. [PubMed] [Google Scholar]
- 30.Wessells K.R., Brown K.H. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hess S.Y. National risk of zinc deficiency as estimated by national surveys. Food Nutr. Bull. 2017;38(1):3–17. doi: 10.1177/0379572116689000. [DOI] [PubMed] [Google Scholar]
- 32.Wuehler S.E., Peerson J.M., Brown K.H. Use of national food balance data to estimate the adequacy of zinc in national food supplies: methodology and regional estimates. Public Health Nutr. 2005;8(7):812–819. doi: 10.1079/phn2005724. [DOI] [PubMed] [Google Scholar]
- 33.Gibson R.S., Hess S.Y., Hotz C., Brown K.H. Indicators of zinc status at the population level: a review of the evidence. Br. J. Nutr. 2008;99(S3):S14–S23. doi: 10.1017/S0007114508006818. [DOI] [PubMed] [Google Scholar]
- 34.Gibson R.S. Zinc: the missing link in combating micronutrient malnutrition in developing countries. Proc. Nutr. Soc. 2006;65(1):51–60. doi: 10.1079/pns2005474. [DOI] [PubMed] [Google Scholar]
- 35.Lowe N.M. Assessing zinc in humans. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19(5):321–327. doi: 10.1097/MCO.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 36.Prasad A.S. Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. Elsevier; 2017. Discovery of zinc for human health and biomarkers of zinc deficiency; pp. 241–260. [Google Scholar]
- 37.Trame S., Wessels I., Haase H., Rink L. A short 18 items food frequency questionnaire biochemically validated to estimate zinc status in humans. J. Trace Elem. Med. Biol. 2018;49:285–295. doi: 10.1016/j.jtemb.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Fraker P.J., King L.E. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 39.Aydemir T.B., Blanchard R.K., Cousins R.J. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc. Natl. Acad. Sci. U. S. A. 2006;103(6):1699–1704. doi: 10.1073/pnas.0510407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., Svistunov A.A., Petrakis D., Spandidos D.A., Aaseth J. Zinc and respiratory tract infections: perspectives for COVID‑19 (Review) Int. J. Mol. Med. 2020 doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., Svistunov A.A., Petrakis D., Spandidos D.A., Aaseth J. International Journal of Molecular Medicine. Spandidos Publications; 2020. Zinc and respiratory tract infections: perspectives for COVID‑19. Permission to reprint Figure 1; pp. 17–26. Edited by COVID-19. PtrF-Tppmozi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han Y.-S., Chang G.-G., Juo C.-G., Lee H.-J., Yeh S.-H., Hsu J.T.-A., Chen X. Papain-like protease 2 (PLP2) from severe acute respiratory syndrome coronavirus (SARS-CoV): expression, purification, characterization, and inhibition. Biochemistry. 2005;44(30):10349–10359. doi: 10.1021/bi0504761. [DOI] [PubMed] [Google Scholar]
- 43.te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scholz M., Derwand R. 2020. Does Zinc Supplementation Enhance the Clinical Efficacy of Chloroquine/Hydroxychloroquine to Win Todays Battle Against COVID-19? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bao S., Knoell D.L. Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006;291(6):L1132–L1141. doi: 10.1152/ajplung.00207.2006. [DOI] [PubMed] [Google Scholar]
- 46.Bao S., Knoell D.L. Zinc modulates airway epithelium susceptibility to death receptor-mediated apoptosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006;290(3):L433–L441. doi: 10.1152/ajplung.00341.2005. [DOI] [PubMed] [Google Scholar]
- 47.Truong-Tran A.Q., Grosser D., Ruffin R.E., Murgia C., Zalewski P.D. Apoptosis in the normal and inflamed airway epithelium: role of zinc in epithelial protection and procaspase-3 regulation. Biochem. Pharmacol. 2003;66(8):1459–1468. doi: 10.1016/s0006-2952(03)00498-2. [DOI] [PubMed] [Google Scholar]
- 48.Truong‐Tran A.Q., Carter J., Ruffin R., Zalewski P.D. New insights into the role of zinc in the respiratory epithelium. Immunol. Cell Biol. 2001;79(2):170–177. doi: 10.1046/j.1440-1711.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 49.Zalewski P.D., Truong-Tran A.Q., Grosser D., Jayaram L., Murgia C., Ruffin R.E. Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical _targets. A review. Pharmacol. Ther. 2005;105(2):127–149. doi: 10.1016/j.pharmthera.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Knoell D.L., Liu M.-J. Impact of zinc metabolism on innate immune function in the setting of sepsis. Int. J. Vitam. Nutr. Res. 2010;80(4):271. doi: 10.1024/0300-9831/a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad A.S. Zinc: mechanisms of host defense. J. Nutr. 2007;137(5):1345–1349. doi: 10.1093/jn/137.5.1345. [DOI] [PubMed] [Google Scholar]
- 52.Boron B., Hupert J., Barch D.H., Fox C.C., Friedman H., Layden T.J., Mobarhan S. Effect of zinc deficiency on hepatic enzymes regulating vitamin A status. J. Nutr. 1988;118(8):995–1001. doi: 10.1093/jn/118.8.995. [DOI] [PubMed] [Google Scholar]
- 53.Mammadova-Bach E., Braun A. Zinc homeostasis in platelet-related diseases. Int. J. Mol. Sci. 2019;20(21) doi: 10.3390/ijms20215258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vu T.T., Fredenburgh J.C., Weitz J.I. Zinc: an important cofactor in haemostasis and thrombosis. Thromb. Haemost. 2013;109(3):421–430. doi: 10.1160/TH12-07-0465. [DOI] [PubMed] [Google Scholar]
- 55.López-Alcalde J., Yan Y., Witt C.M., Barth J. 2020. Complementary, Alternative and Integrative Medicine (CAM) for the Treatment of Coronavirus Disease 2019 (COVID-19): an Overview: Licensed Under a Creative Commons Attribution 4.0 International License. [Google Scholar]
- 56.Marshall I. Zinc for the common cold. Cochrane Database Syst. Rev. 2000;(2) doi: 10.1002/14651858.CD001364. Cd001364. [DOI] [PubMed] [Google Scholar]
- 57.Lassi Z.S., Moin A., Bhutta Z.A. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst. Rev. 2016;12 doi: 10.1002/14651858.CD005978.pub3. Cd005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hulisz D. Efficacy of zinc against common cold viruses: an overview. J. Am. Pharm. Assoc. 2004;44(5):594–603. doi: 10.1331/1544-3191.44.5.594.Hulisz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson J.L., Lesho E., Peterson C. Zinc and the common cold: a meta-analysis revisited. J. Nutr. 2000;130(5S Suppl):1512s–1515s. doi: 10.1093/jn/130.5.1512S. [DOI] [PubMed] [Google Scholar]
- 60.Hemilä H. Zinc lozenges may shorten the duration of colds: a systematic review. Open Respir. Med. J. 2011;5:51. doi: 10.2174/1874306401105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hemila H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 2017;8(5) doi: 10.1177/2054270417694291. 2054270417694291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh M., Das R.R. Zinc for the common cold. Cochrane Database Syst. Rev. 2013;(6) doi: 10.1002/14651858.CD001364.pub4. Cd001364. [DOI] [PubMed] [Google Scholar]
- 63.Singh M., Das R.R. WITHDRAWN: zinc for the common cold. Cochrane Database Syst. Rev. 2015;(4) doi: 10.1002/14651858.CD001364.pub5. Cd001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hemilä H., Chalker E. Zinc for preventing and treating the common cold [Protocol Withdrawn] Cochrane Database Syst. Rev. 2019;(11) doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.PROSPERO; 2020. Protocol for a Rapid Review of Zinc for the Prevention or Treatment of COVID-19 and Other Coronavirusrelated Respiratory Tract Infections in Humans.www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020182044 CRD42020182044. [Google Scholar]
- 66.Sterne J.A., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.-Y., Corbett M.S., Eldridge S.M. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 67.Higgins J.P., Sterne J.A., Savovic J., Page M.J., Hróbjartsson A., Boutron I., Reeves B., Eldridge S. Revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst. Rev. 2016;10(Suppl 1):24. [Google Scholar]
- 68.Navarro V., Avula B., Khan I., Verma M., Seeff L., Serrano J., Stolz A., Fontana R., Ahmad J. The contents of herbal and dietary supplements implicated in liver injury in the United States are frequently mislabeled. Hepatol. Commun. 2019;3(6):792–794. doi: 10.1002/hep4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Few-Clinical Trials [https://osf.io/qw54t/wiki/Few-Clinical-Trials/].
- 70.Ischia J. In: HDIVZn Trial Progress. Hunter J., editor. 2020. [Google Scholar]
- 71.Greenberg S.B. Seminars in Respiratory and Critical Care Medicine: 2016. Thieme Medical Publishers; 2016. Update on human rhinovirus and coronavirus infections; pp. 555–571. [DOI] [PMC free article] [PubMed] [Google Scholar]