Abstract
Glutamatergic dysregulation is known to contribute to obsessive-compulsive disorder (OCD). Astrocytic glutamate transporter 1 (GLT1) is responsible for the majority of glutamate clearance. However, the role of GLT1 in OCD-like behavior remains unclear. Here, we found that astrocytic GLT1 deficient mice showed increased wheel running activity but reduced home cage activity. Notably, they exhibited elevated grooming/rearing time and increased repetitive behavior counts in contextual and cued fear conditioning. In addition, they showed increased rearing counts in the metabolic chamber, and also augmented rearing time and jumping counts in the open field test. Taken together, our findings suggest that astrocytic GLT1 deficiency promotes OCD-like repetitive behaviors.
Keywords: Glutamate transporter 1 (GLT1), Obsessive-compulsive disorder (OCD), Repetitive behavior, grooming, Wheel running activity, Fear conditioning
1. Introduction
Glutamatergic dysregulation is known to be essential in obsessive-compulsive disorder (OCD) as elevated glutamate levels are found in OCD patients [1]. The glutamatergic pathophysiology of OCD has also been reported in animal studies [2, 3] as well as with pediatric OCD patients utilizing MRS-imaging [4–6]. In particular, abnormal repetitive behaviors, including climbing and leaping, can be aggravated by stimulating cortical-limbic glutamate output in a transgenic mouse model of comorbid Tourette’s syndrome and OCD [2]. Similarly, striatal glutamatergic neurotransmission is associated with increased self-grooming in animal models of autism spectrum disorder (ASD), OCD, and Tourette’s syndrome [7–10].
In the central nervous system (CNS), extracellular and synaptic glutamate levels are regulated by the glutamate/neutral amino acid transporters (EAATs), which is also known as solute carrier family 1 (SLC1) transporters [11]. Among five well-characterized EAATs, EAAT2 glutamate transporter 1 (GLT1 or a.k.a. EAAT2) and glutamate transporter 3 (EAAT3) are associated with pathophysiology of OCD [12, 13]. Especially, GLT1 is responsible for the majority (>90%) of glutamate clearance [14]. Global GLT1 null mice showed high glutamate levels in the brain and exhibited sever seizure activity with lethal spontaneous seizures and increased susceptibility to acute cortical injury [15]. Interestingly, astrocytic-specific, not neuronal-specific, deletion of GLT1 induced fatal epilepsy, suggesting that astrocytic GLT1 plays a critical role in epilepsy and survival [16]. It is believed that a slight elevation to glutamate levels may promote repetitive behaviors, whereas greater glutamate elevations more readily induce stereotypes and limbic seizure behaviors [1, 2].
Interestingly, inducible conditional knockout of GLT1 in astrocytes displayed pathological repetitive behaviors including excessive self-grooming and tic-like head shakes [17], which implicated that GLT1 is involved in pathological regulation of OCD symptoms. As summarized [13], several repetitive behaviors were frequently observed in typical OCD mouse models including mice lacking Spred2 (Sprouty-related, EVH1 domain-containing protein 2) [18], Sapap3 (SAP90/PSD95-associated protein 3)[19] or Slitrk5 (SLIT and NTRK-like protein-5) [20]. These findings suggested that GLT1 may account for repetitive behavior and subsequent seizure activity. To study OCD-like repetitive behavior, perseverance or repetition of normal behaviors such as grooming, rearing, and body licking are used to characterize the performance of animals [7, 21]. As a genetic model of OCD, aromatase KO mice exhibited increased wheel-running activity and grooming but decreased ambulatory activity [22]. Therefore, we utilized wheel running, home cage activity, and rearing/jumping in this study as behavioral indicators to investigate repetitive behaviors in astrocytic GLT1 deficient mice.
A number of studies have demonstrated the association of GLT1 and glutamate clearance dysregulation to the pathophysiology of OCD and fear response [1, 2, 4–6, 23]. Recent translational research suggests that dysfunctional fear acquisition and extinction learning may be at the core of many anxiety disorders including OCD [24].
Since the role of astrocytic GLT1 in repetitive behaviors and fear conditioning remains largely unknown, we conducted this study aiming to explore the effects of astrocytic GLT1 deletion on repetitive behaviors using well-established OCD-like behavioral tests, including wheel running, home cage activity, rearing and jumping, as well as on fear conditioning responses. In our study, we revealed a novel aspect of astrocytic GLT1 in OCD-like behaviors.
2. Materials and methods
2.1. Animals
We used the same mice as described previously [25]. Eight-week old male mice were group-housed (4–5 animals per cage) in standard Plexiglas cages in a 12 h light/dark cycle (lights on at 6 AM and off at 6 PM) with a temperature (22–24°C) and humidity (50%) regulated environment with access to standard lab food and water ad libitum. The floxed-GLT1 mice (GLT1F/F) were obtained from Niels C. Danbolt’s laboratory (University of Oslo, Norway) [26] and the GFAPcre/+ line was purchased from the Jackson laboratory [Cat no., 024098 - B6.Cg-Tg(Gfap-cre)77.6Mvs/2J]. To generate astrocyte-specific GLT1 knockout mice, we crossed the GLT1F/F mice with the GFAPcre/+ line, in which Cre recombinase is expressed selectively in the astrocytes. Previously [25], we confirmed the near-complete deletion of GLT1 in GFAP-positive cells in the mPFC, striatum and hippocampus. As we demonstrated with co-immunostaining with neuronal marker (anti-NeuN antibody), the remaining protein and mRNA GLT1 expression mainly reflects the neuronal GLT1 expression. All mice in this study had C57BL/6J genetic background and a total of 63 mice were used (GFAPcre/−;GLT1F/F n = 32 and GFAPcre/+; GLT1F/F n = 31). All animal care, handling procedures and experimental protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC) in accordance with the guidelines set forth by the National Institutes of Health.
2.2. Behavioral tests
Mice were acclimated to the testing room for 30 minutes prior to each behavioral test. Four independent cohorts were used for wheel running /clocklab homecage activity tests, fear conditioning tests, open field, and metabolic chamber tests. For the survival statistics, mice were observed from the fear conditioning tests, open field, and metabolic chamber tests (GFAPcre/−;GLT1F/F n = 25 and GFAPcre/+; GLT1F/F n = 23).
2.2.1. Wheel running test
Mice were individually housed and placed in a home cage consisting of a wheel running activity monitor interfaced with Clocklab (Coulbourn Instruments, Whitehall, PA). Mice were housed in a 12 h light/dark cycle (lights on at 6 AM and off at 6 PM) with free access to food and water. Wheel running activity counts consisted of a turn of the wheel by the mouse and averaged over 12 days. Total activity counts of (activity amplitude), counts during the active phase (dark period), and counts during the rest phase (light period) were obtained from the daily average of 12 consecutive days per 24 h.
2.2.2. Clocklab homecage activity test
The home cage activity [27] was monitored for 12 consecutive days by infrared sensors interfaced with Clocklab (Coulbourn Instruments, Whitehall, PA) while the mice were individually caged. Mice were housed in a 12 h light/dark cycle (lights on at 6 AM and off at 6 PM) with free access to food and water. Homecage activity counts were averaged over the 12 days for each mouse for total activity counts (Activity amplitude), counts during the active phase (dark period), and counts during the rest phase (light period).
2.2.3. Grooming and rearing behaviors in fear conditioning test
The fear conditioning test was performed as we described previously [25]. In the meantime, we observed the repetitive behaviors such as grooming and rearing in the fear memory test periods (on day 2 and day 3). Briefly, the behavior was recorded using a high-speed fire wire monochrome video camera with a near infrared pass filter on an 8 mm lens and analyzed using Video Freeze® software (Med Associates). On day 1, mice were trained with 5 pairings of 30 s tone (75 dB, 3000 kHz) with a shock occurring the last 2 s of the tone (0.4 mA) and a 2-min inter-trial interval (ITI 2 min). On day 2 for the contextual test, mice were only presented the same context (no sound, no shock). On day 3 for the cued test, a white floor grid cover and a black A-frame chamber insert were added to the chamber. Mice were subjected to 5 presentations of 30 s tones (75 dB, 3000 kHz) without a shock at a 2-min inter-trial interval (ITI 2 min).
Grooming behavior included face-wiping, full-body grooming, and scratching and rubbing of head and ears [18, 21]. The total amount of time spent in grooming, and the number of grooming and rearing were manually measured during fear condition contextual and cued tests, respectively. The chamber was cleaned with 70% ethanol and allowed to dry completely prior to testing in between each animal.
2.2.4. Open field test (OFT)
The ENV-510 test environment equipped with infrared beams and Activity Monitor (Med Associates) were used to evaluate motor activity in the open field test. Mice were placed in a Plexiglas box (27 × 27 × 20.3 cm) and allowed to explore the chamber for 10 min. The data was recorded by each beam break as one unit of exploratory activity using the activity monitoring software (Med Associates).
2.2.5. Metabolic chamber
As described [25], mice were placed in the metabolic cages (Oxylet Pro, PanLab) for 24 h for habituation, followed by 48 h to measure oxygen consumption and energy expenditure. Mice were maintained at a 12 h light/dark cycle with lights on at 6 AM and off at 6 PM. Mice were free to access food and water. Rearing counts, energy expenditure, volume of CO2 (vCO2), and volume of O2 (vO2) were obtained using a gas analyzer (Panlab, LE 405 Gas Analyzer) and metabolism software (Panlab, Metabolism).
2.3. Statistical analysis
All data are expressed as mean ± SEM (standard error of the mean). Analyses were conducted using GraphPad Prism (version 6.0). Unpaired two-tailed Student’s t-test was used to compare the difference between two groups. Two-way repeated measures ANOVA was used to detect the effects of time and genotypes. ANOVA were followed by Tukey post hoc tests where interactions were significant. Statistical significance was set at P < 0.05.
3. Results
3.1. Astrocytic GLT1 deficiency potentiates repetitive behavior in wheel-running test
We generated astrocyte-specific GLT1 knockout mice by crossing the floxed-GLT1 mice (GLT1F/F) with the GFAPcre/+ line, in which Cre recombinase was expressed selectively in the astrocytes. Western blot, quantitative real-time PCR (qRT-PCR), and immunohistochemical analyses of GLT1 revealed a significant reduction of GLT1 protein and mRNA in the brain of 8-week-old GFAPcre/+;GLT1F/F mice [25]. As global GLT1 knockout mice show increased mortality [15], we measured lifespan up to 6 months in GFAPcre/+;GLT1F/F mice. The survival rates of GFAPcre/+;GLT1F/F mice and controls (GLT1F/F mice) were indistinguishable up to 8 weeks (2.5 months). However, we found a notable decline in life span from 2.5 months to 6 months (Fig. S1). We excluded any mice from the following behavioral analysis once they showed severe seizure-like phenotypes.
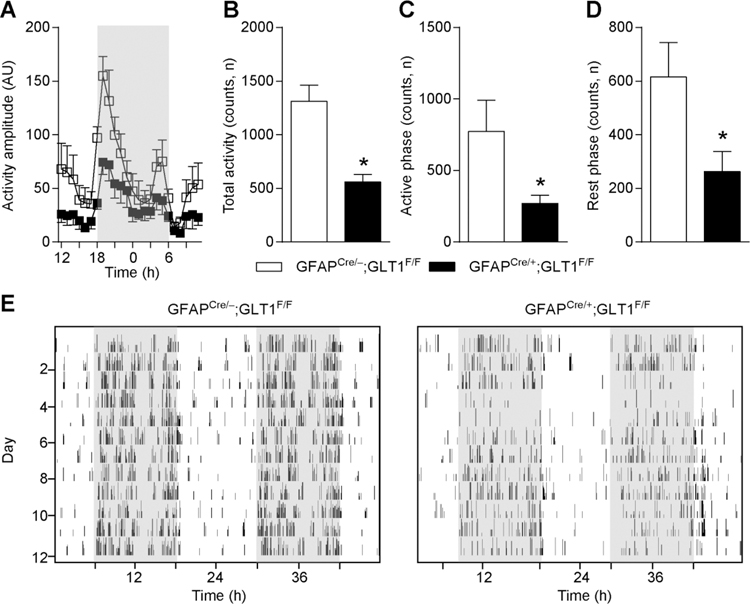
In order to examine voluntary compulsive behavior in mice lacking astrocytic GLT1, we utilized a wheel running test [28, 29]. Each mouse was housed in a home cage installed with a running wheel for 12 consecutive days. We found that GFAPcre/+;GLT1F/F mice showed a significant increase of activity amplitude (Two-way ANOVA; group effect: F 1, 8= 7.662, p=0.024, time effect: F 23, 184= 17.453 p<0.0001, interaction: F 23, 184= 2.298, p=0.02) compared to controls during the entire time (Fig. 1A–E). The total activity counts were significantly increased (two-tailed unpaired t test; t7=2.99, p=0.02) in GFAPcre/+;GLT1F/F mice (Fig. 1B). Both active (dark period) phase activity (two-tailed unpaired t test; t7=2.45, p=0.04, Fig. 1C) and resting (light period) phase activity (two-tailed unpaired t test; t7= 2.68, p=0.03, Fig. 1D) were significantly increased in GFAPcre/+;GLT1F/F mice compared to controls (GFAPcre/−;GLT1F/F). The representative actograms were shown as Fig. 1E.
Fig. 1.
Prolonged activity in wheel running test in GFAPcre/+;GLT1F/F mice. (A) Activity amplitude of wheel running test in GFAPcre/+;GLT1F/F mice (n = 6) and controls (n = 4). Amplitude was measured by arbitrary unit (AU). (B) Total activity counts of wheel running test in GFAPcre/+;GLT1F/F mice (n = 5) and controls (n = 4). (C) Activity counts during active phase (dark period) of wheel running test in GFAPcre/+;GLT1F/F mice (n = 5) and controls (n = 4). (D) Activity counts during rest phase (light period) of wheel running test in GFAPcre/+;GLT1F/F mice (n = 5) and controls (n = 4). (E) Representative images of actogram for GFAPcre/+;GLT1F/F mice and control. All data are presented as mean ± SEM. Statistical significance was calculated by Student’s t-test in (B, C, and D), and by two-way repeated measures ANOVA with post hoc t-test for multiple comparisons in (A). *P < 0.05.
To quantify home cage activity without a wheel, we used Clocklab system with a motion monitor sensor installed on the cage lid to monitor home cage activity continuously for 12 days. We found that the activity amplitude in GFAPcre/+;GLT1F/F mice was significantly lower (Two-way ANOVA; group effect: F 1, 13= 13.16, p< 0.003, time effect: F 23, 299= 7.826, p<0.0001, interaction: F 23, 299= 1.124, p=0.317) than that of control mice (Fig. 2A–E), as well as the total activity counts (two-tailed unpaired t test; t10= 3.966, p=0.002, Fig. 2B). Both active phase activity (two-tailed unpaired t test; t12=2.213, p=0.047; dark period, Fig. 2C) and resting phase activity (two-tailed unpaired t test; t10= 2.383, p=0.038; light period, Fig. 2D) were significantly reduced in GFAPcre/+;GLT1F/F mice compared to the controls. These results indicated that astrocytic deletion of GLT1 reduced home cage activity. The representative actograms were shown as Fig. 2E.
Fig. 2.
Lower activity in Clocklab homecage monitor test in GFAPcre/+;GLT1F/F mice. (A) Activity amplitude in home cage by Clocklab system monitor sensor assembled on the lid in GFAPcre/+;GLT1F/F mice (n = 8) and controls (n = 7). (B) Total activity counts of GFAPcre/+;GLT1F/F mice (n = 5) and controls (n = 7) in home cage. (C) Active phase activity counts (dark period) of GFAPcre/+;GLT1F/F mice (n = 7) and controls (n = 7) in home cage. (D) Rest phase activity counts (light period) of GFAPcre/+;GLT1F/F mice (n = 6) and controls (n = 6) in home cage. (E) Representative images of actogram for GFAPcre/+;GLT1F/F mice and control. All data are presented as mean±SEM. All data are presented as mean ± SEM. Statistical significance was calculated by Student’s t-test in (B, C, and D), and by two-way repeated measures ANOVA with post hoc t-test for multiple comparisons in (A). *P < 0.05.
3.2. Excessive repetitive behavior was found in fear conditioning chamber of astrocytic GLT1 deficient mice
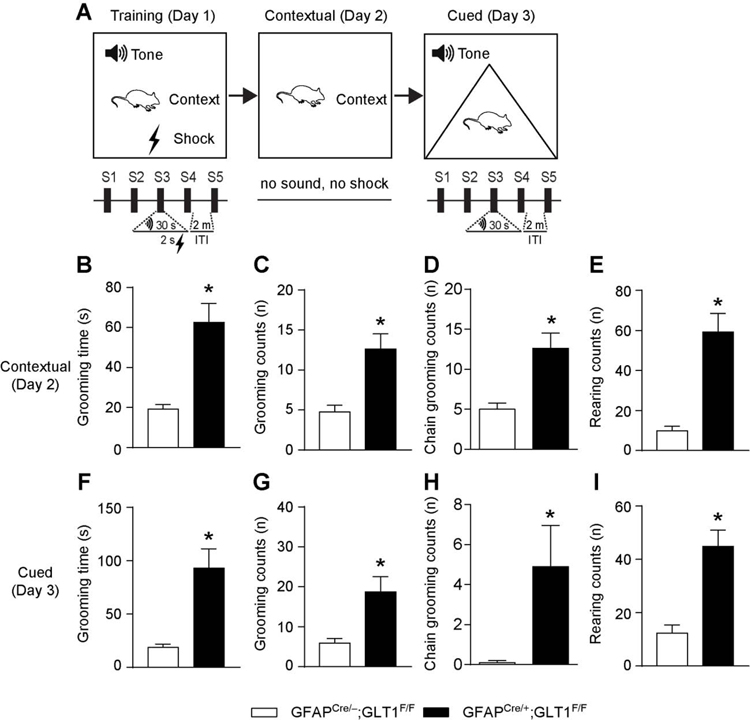
Accurate associative fear memories are important to avoid harmful stimuli. Impaired fear response is a high-risk factor of OCD [30–33]. To examine the role of astrocytic GLT1 in fear conditioning, we trained mice with 5 tone/foot shock pairing on day 1, and then we performed the contextual (day 2) and cued (day 3) fear memory tests on the following days (Fig. 3A). Previously, we showed that GFAPcre/+;GLT1F/F mice exhibited comparable freezing responses across the training trials but decreased freezing response compared to control mice [25]. In this study, we examined the repetitive behavioral patterns including grooming and rearing behaviors during the contextual and cued fear memory tests. As expected, in the contextual test, GFAPcre/+;GLT1F/F mice exhibited more grooming time (two-tailed unpaired t test; t15= 4.713, p= 0.0003; Fig. 3B) and grooming counts (two-tailed unpaired t test; t15= 3.891, p= 0.001; Fig. 3C). Mouse self-grooming has a complex sequenced structure that consists of repeated stereotyped movements known as syntactic chains grooming [7]. Importantly, we found more chains grooming in GFAPcre/+;GLT1F/F mice than controls in the contextual test (two-tailed unpaired t test; t15= 2.221, p= 0.04; Fig.3D). In addition, they also showed more rearing counts (two-tailed unpaired t test; t15= 5.537, p< 0.001; Fig. 3E) than controls. Similarly, in the cued test, GFAPcre/+;GLT1F/F mice had more grooming time (two-tailed unpaired t test; t18= 4.074, p= 0.0007; Fig. 3F), grooming counts (two-tailed unpaired t test; t18= 3.181, p= 0.005; Fig. 3G), chains grooming counts (two-tailed unpaired t test; t18= 2.337, p= 0.031; Fig. 3H), as well as more rearing counts (two-tailed unpaired t test; t18= 4.805, p= 0.0001; Fig. 3I) compared to controls [see representative videos for 2 min of repetitive behavior during contextual (Video S1–S2) and cued (Video S3–S4) tests as a supplementary]. These results suggested that deletion of astrocytic GLT1 is associated with the excessive grooming and rearing behaviors.
Fig. 3.
Excessive repetitive behavior in fear memory test in GFAPcre/+;GLT1F/F mice. (A) The experimental schedule for performing fear conditioning test. (B) The grooming time during contextual test of GFAPcre/+;GLT1F/F mice (n = 8) and controls (n = 9). (C) The total counts of grooming behavior during contextual test of GFAPcre/+;GLT1F/F mice (n = 8) and controls (n = 9). (D) The total counts of syntactic chains grooming during contextual test of GFAPcre/+;GLT1F/F mice (n = 8) and controls (n = 9). (E) The total counts of rearing behavior during contextual test of GFAPcre/+;GLT1F/F mice (n = 8) and controls (n = 9). (F) The grooming time during cued test of GFAPcre/+;GLT1F/F mice (n = 10) and controls (n = 10). (G) The total counts of grooming behavior during cued test of GFAPcre/+;GLT1F/F mice (n = 10) and controls (n = 10). (H) The total counts of syntactic chains grooming during cued test of GFAPcre/+;GLT1F/F mice (n = 10) and controls (n = 10). (I) The total counts of rearing behavior during cued test of GFAPcre/+;GLT1F/F mice (n = 10) and controls (n = 10). All data are presented as mean ± SEM. Statistical significance was calculated by Student’s t-test. *P < 0.05.
3.3. Repetitive behavior was found in open-field test of astrocytic GLT1 deficient mice without altering caloric consumptions
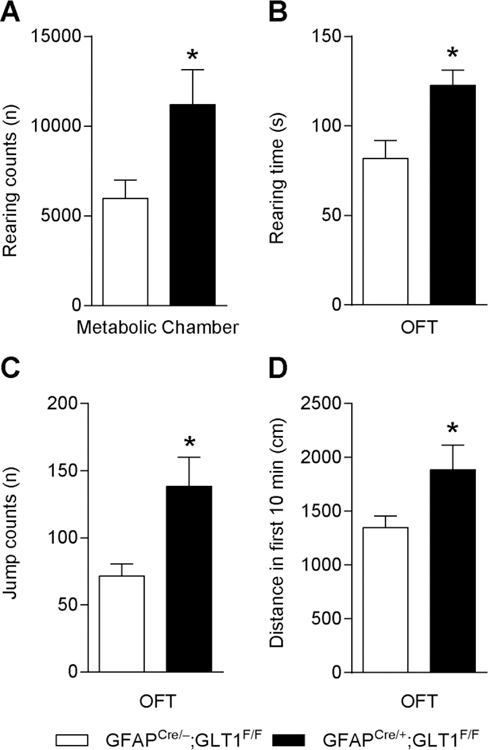
Next, we examined whether the basal metabolic activity was affected by the deletion of GLT1 in astrocytes, as assessed by metabolic chamber for a continuous 48 hours. Interestingly, we found that GFAPcre/+;GLT1F/F mice exhibited increased rearing counts than controls (two-tailed unpaired t test; t4= 2.859, p= 0.046; Fig. 4A), while the energy expenditure, volume of CO2, and volume of O2 metabolism were not changed [25].
Fig. 4.
Persistent behavior of GFAPcre/+;GLT1F/F mice in metabolic and open field chambers. (A) Total counts of rearing behavior for 48 h in GFAPcre/+;GLT1F/F mice (n = 3) and controls (n = 3) in the metabolic chamber. (B) Duration of rearing behavior in GFAPcre/+;GLT1F/F mice (n = 10) and controls (n = 12) in the open field test. (C) Jump counts in GFAPcre/+;GLT1F/F mice (n = 10) and controls (n = 11) in the open field test. (D) Distance moved in the open field of GFAPcre/+;GLT1F/F mice (n = 9) and controls (n = 9). All data are presented as mean±SEM. Statistical significance was calculated by Student’s t-test. *P < 0.05.
In order to investigate locomotor activity in a novel environment, we employed the open field test. GFAPcre/+;GLT1F/F mice showed an increase in both rearing time (two-tailed unpaired t test; t19= 3.091, p= 0.006; Fig. 4B) and jump counts (two-tailed unpaired t test; t19= 2.952, p=0.008,Fig. 4C) in the 10 min of the open field compared to controls. In addition, the distance travelled was also increased in the GFAPcre/+;GLT1F/F mice compared to controls (two-tailed unpaired t test; t16= 2.136, p=0.048,Fig. 4D). Taken together, our results showed a repetitive behavior pattern in running activity in the wheel running test, and elevated rearing time, jump counts, distance in the earlier stage of open field test, as well as increased rearing times in the metabolic chamber.
4. Discussion
The present study demonstrates that astrocyte-specific deletion of GLT1 promotes OCD-like repetitive behaviors using a comprehensive array of behavioral tests including compulsive running, and excessive grooming and rearing behaviors. This may result from glutamatergic dysregulation, as GLT1 accounting for more than 90% of glutamate uptake in the forebrain [15].
Astrocytic GLT1 deficiency increases repetitive behaviors showing excessive grooming and tic-like movements [17], which were consistent with the increased grooming and rearing behaviors during the fear conditioning contextual and cued tests in our current study. Importantly, our findings provide a novel aspect of how astrocytic deficient GLT1 mice had an increase of syntactic chains grooming, which is frequently lost during stress and anxiety in wild type mice [7]. Striatal activity is required for syntactic chains grooming behavior, as lesions of the striatum result in a deficiency in the ability to complete sequential syntactic self-grooming chains [34]. Dopamine transporter-deficient mice with an elevated dopamine levels exhibit more predictable syntactic grooming sequences than controls [35]. As another excitatory neurotransmitter, glutamate function was unknown in syntactic chains grooming behaviors. Therefore, the contribution of striatal astrocytic GLT1 and/or glutamate function in OCD and specifically syntactic chains grooming behaviors is warranted for the future study.
Aromatase KO mice, as a genetic model of OCD, showed increased wheel-running activity and grooming but decreased ambulatory activity [21, 22]. Similarly, we showed the increased wheel running activity, which is believed as a type of compulsive running behavior of OCD [29]. Additionally, the repetitive behaviors were also observed as elevated rearing time and jump counts (repetitive behavior) in the open field test, as well as increased rearing counts in metabolic chamber, which further supported our notion that astrocytic GLT1 accounts for repetitive behavior. The excessive repetitive behaviors in astrocytic GLT1 deficiency might be caused by glutamatergic hyperactivity [3, 17] since the high glutamate level was found in mice with deletion of GLT1 either global or astrocytic [15, 16]. Despite of hyperactivity, the energy expenditures were similar between GFAPcre/+;GLT1F/F mice and control mice [25]. Although this is an unexpected result, since the overall home cage activity was reduced, tracked by the Clocklab system monitor, which might explain partially the counter-balancing of energy expenditures during non-stimulus environments.
As a typical OCD-like behavior transgenic mouse model, the Spred2 deficient mice showed reduced anxiety-like behaviors [18]. Notably, our previous findings [25] also showed that astrocytic GLT1 deficiency seemed to show less anxiety in the elevated plus maze, which is consistent with Spred2-deficient mouse model of OCD [18]. Interestingly, Spred2-deficient mice showed increased protein expression related to glutamate signaling such as PSD95, mGluR2 and 5 in the amygdala [18]. Since the blockade of GLT1 in the amygdala induces anxiety- and depression-like behaviors in mice [36], the aberrant glutamate neurotransmission in the amygdala circuits may be the common denominators of these two animal models. The increased locomotor activity observed in the open field test and elevated plus maze also supports that GLT1 deletion in the astrocyte might induce repetitive behavior. However, they travelled more distance in the entire maze and had more open arm entries, which might be a sign that they were repetitively checking the new environment. There is one study reported that GLT1 was involved in OCD without affecting anxiety-like behavior [17], a contradictory finding from the OCD mouse model. Overall, the contribution of GLT1 in regard to OCD, anxiety, and depressive behavior remains highly controversial. It has been previously reported that administration with GLT1-selective inhibitor dihydrokainate (DHK) induces robust antidepressant-like responses [37], which is consistent with our previous findings [25] of tail suspension and forced swim tests with mice displaying less immobile time revealed in the astrocytic deletion of GLT1. However, it is possible that less immobility (more movement) might be an indicator of repetitive behaviors in a short period.
In clinical settings, OCD patients exhibit sensitive reaction to the presentations of novel stimuli, which are related to early attention biases to threat [38, 39]. These behaviors were reflected in the enhanced locomotor activities in astrocytic GLT1 deficient mice when they access a new environment such as open field and elevated plus maze in our studies. Notably, the impairment of fear memory is a predisposition of OCD. The mechanisms of fear acquisition and extinction play important roles in symptom development, maintenance and treatment of OCD [40]. It has been previously shown that anxiety and stress promotes and increases repetitive or ritualized behaviors or tics in humans [41, 42] as well as in animal models of ASD, OCD and Tourette’s syndrome [7, 10, 35, 43, 44]. The excessive grooming and rearing behaviors revealed in astrocytic GLT1 deficient mice are associated with the reduced fear response in the fear memory test period, suggesting that reduced fear memory may be a predictor of OCD-like repetitive behaviors.
In addition, Petr et.al., [16] reported that only astrocytic (not neuronal) deletion of GLT1 induced seizure activity, which suggested that the pathological repetitive behavior was due to glial dysfunction particularly important in the pathophysiology of excessive repetitive behavior. Furthermore, recent data indicated that selective deletion of GLT1 in the diencephalon, brainstem and spinal cord was sufficient to reproduce the phenotypes (excess mortality, decreased body weight, and lethal spontaneous seizure) [45]. Likewise, GLT1 dysfunction in the dorsal forebrain is involved in the pathogenesis of infantile epilepsy [45]. Interestingly, we also observed seizure behavior in about 30% of astrocytic GLT1 deficient mice when they reached 10 weeks old. In order to prevent confounding effects on our behavioral analysis, we exclude those mice showing seizure-like behaviors. To clarify temporal spatial role of GLT1 in astrocytes, comprehensive reverse-engineering mechanistic approaches are required.
In summary, our findings suggest that astrocyte-specific deletion of GLT1 promotes obsessive compulsive disorder (OCD)-like repetitive behaviors. Additionally, we are the first to report that excessive repetitive behavior is associated with reduced fear response, which might be a contributor to OCD. Our study reveals a novel role of astrocytic GLT1 in OCD, which may provide a useful therapeutic _target toward the treatment of OCD-like repetitive behaviors.
Supplementary Material
Highlights.
Astrocytic GLT1 deficiency potentiates total locomotor activity in wheel-running test.
Astrocytic GLT1 deficient mice exhibited enhanced grooming and rearing behaviors in fear conditioning chamber.
Astrocytic GLT1 deficient mice showed excessive repetitive behavior in open-field test and metabolic chamber.
Acknowledgements
We thank all members of the DSC.’s laboratory for interest, help and comments. We thank Dr. Niels C. Danbolt for providing GLTF/F mice. We also thank summer undergraduate students, Nicoli Carneiro and Joyce Yang. This work was funded by the Samuel C. Johnson for Genomics of Addiction Program at Mayo Clinic, the Ulm Foundation, and National Institute on Alcohol Abuse and Alcoholism (AA018779).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. DS Choi is a scientific advisory board member to Peptron Inc. and the Peptron had no role in the preparation, review, or approval of the manuscript; nor the decision to submit the manuscript for publication. All the other authors declare no biomedical financial interests or potential conflicts of interest.
References
- [1].Chakrabarty K, Bhattacharyya S, Christopher R, Khanna S, Glutamatergic dysfunction in OCD, Neuropsychopharmacology 30(9) (2005) 1735–40. [DOI] [PubMed] [Google Scholar]
- [2].McGrath MJ, Campbell KM, Parks CR, Burton FH, Glutamatergic drugs exacerbate symptomatic behavior in a transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder, Brain research 877(1) (2000) 23–30. [DOI] [PubMed] [Google Scholar]
- [3].Katz M, Corson F, Keil W, Singhal A, Bae A, Lu Y, Liang YP, Shaham S, Glutamate spillover in C. elegans triggers repetitive behavior through presynaptic activation of MGL-2/mGluR5, Nature Communications 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moore GJ, MacMaster FP, Stewart C, Rosenberg DR, Case study: caudate glutamatergic changes with paroxetine therapy for pediatric obsessive-compulsive disorder, Journal of the American Academy of Child and Adolescent Psychiatry 37(6) (1998) 663–7. [DOI] [PubMed] [Google Scholar]
- [5].Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ, Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine, Journal of the American Academy of Child and Adolescent Psychiatry 39(9) (2000) 1096–1103. [DOI] [PubMed] [Google Scholar]
- [6].Bolton JM, Moore GJ, MacMillan SN, Stewart CM, Rosenberg DR, Case study: Caudate glutamatergic changes with paroxetine therapy for pediatric obsessive-compulsive disorder persist after medication discontinuation, Biological Psychiatry 49(8) (2001) 99s–100s. [DOI] [PubMed] [Google Scholar]
- [7].Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC, Neurobiology of rodent self-grooming and its value for translational neuroscience, Nature reviews. Neuroscience 17(1) (2016) 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Silverman JL, Tolu SS, Barkan CL, Crawley JN, Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35(4) (2010) 976–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN, Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism, Science translational medicine 4(131) (2012) 131ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wan Y, Ade KK, Caffall Z, Ilcim Ozlu M, Eroglu C, Feng G, Calakos N, Circuit-selective striatal synaptic dysfunction in the Sapap3 knockout mouse model of obsessive-compulsive disorder, Biological Psychiatry 75(8) (2014) 623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kanai Y, Hediger MA, The glutamate and neutral amino acid transporter family: physiological and pharmacological implications, Eur J Pharmacol 479(1–3) (2003) 237–47. [DOI] [PubMed] [Google Scholar]
- [12].Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL, Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder, Arch Gen Psychiatry 63(7) (2006) 769–76. [DOI] [PubMed] [Google Scholar]
- [13].Escobar AP, Wendland JR, Chavez AE, Moya PR, The Neuronal Glutamate Transporter EAAT3 in Obsessive-Compulsive Disorder, Front Pharmacol 10 (2019) 1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB, Insights into glutamate transport regulation in human astrocytes: Cloning of the promoter for excitatory amino acid transporter 2 (EAAT2), Proceedings of the National Academy of Sciences of the United States of America 100(4) (2003) 1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K, Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1, Science 276(5319) (1997) 1699–702. [DOI] [PubMed] [Google Scholar]
- [16].Petr GT, Sun Y, Frederick NM, Zhou Y, Dhamne SC, Hameed MQ, Miranda C, Bedoya EA, Fischer KD, Armsen W, Wang J, Danbolt NC, Rotenberg A, Aoki CJ, Rosenberg PA, Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes, The Journal of neuroscience : the official journal of the Society for Neuroscience 35(13) (2015) 5187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aida T, Yoshida J, Nomura M, Tanimura A, Iino Y, Soma M, Bai N, Ito Y, Cui W, Aizawa H, Yanagisawa M, Nagai T, Takata N, Tanaka KF, Takayanagi R, Kano M, Gotz M, Hirase H, Tanaka K, Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice, Neuropsychopharmacology 40(7) (2015) 1569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ullrich M, Weber M, Post AM, Popp S, Grein J, Zechner M, Guerrero Gonzalez H, Kreis A, Schmitt AG, Uceyler N, Lesch KP, Schuh K, OCD-like behavior is caused by dysfunction of thalamo-amygdala circuits and upregulated TrkB/ERK-MAPK signaling as a result of SPRED2 deficiency, Molecular psychiatry 23(2) (2018) 444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G, Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice, Nature 448(7156) (2007) 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shmelkov SV, Hormigo A, Jing D, Proenca CC, Bath KG, Milde T, Shmelkov E, Kushner JS, Baljevic M, Dincheva I, Murphy AJ, Valenzuela DM, Gale NW, Yancopoulos GD, Ninan I, Lee FS, Rafii S, Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice, Nature medicine 16(5) (2010) 598–602, 1p following 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alonso P, Lopez-Sola C, Real E, Segalas C, Menchon JM, Animal models of obsessive-compulsive disorder: utility and limitations, Neuropsychiatric disease and treatment 11 (2015) 1939–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hill RA, McInnes KJ, Gong EC, Jones ME, Simpson ER, Boon WC, Estrogen deficient male mice develop compulsive behavior, Biological Psychiatry 61(3) (2007) 359–66. [DOI] [PubMed] [Google Scholar]
- [23].Griffin WC, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC, Increased Extracellular Glutamate In the Nucleus Accumbens Promotes Excessive Ethanol Drinking in Ethanol Dependent Mice, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39(3) (2014) 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Graham BM, Milad MR, The study of fear extinction: implications for anxiety disorders, The American journal of psychiatry 168(12) (2011) 1255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jia YF, Wininger K, Ho AM, Peyton L, Baker M, Choi DS, Astrocytic Glutamate Transporter 1 (GLT1) Deficiency Reduces Anxiety- and Depression-Like Behaviors in Mice, Front Behav Neurosci 14 (2020) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, Carmichael T, Kornblum HI, Liu X, Wu H, Pten Deletion in Adult Neural Stem/Progenitor Cells Enhances Constitutive Neurogenesis, Journal of Neuroscience 29(6) (2009) 1874–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jia YF, Vadnie CA, Ho AM, Peyton L, Veldic M, Wininger K, Matveyenko A, Choi DS, Type 1 equilibrative nucleoside transporter (ENT1) regulates sex-specific ethanol drinking during disruption of circadian rhythms, Addiction biology (2019) e12801. [DOI] [PMC free article] [PubMed]
- [28].Werme M, Thoren P, Olson L, Brene S, Addiction-prone Lewis but not Fischer rats develop compulsive running that coincides with downregulation of nerve growth factor inducible-B and neuron-derived orphan receptor 1, The Journal of neuroscience : the official journal of the Society for Neuroscience 19(14) (1999) 6169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Korff S, Harvey BH, Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology, The Psychiatric clinics of North America 29(2) (2006) 371–90. [DOI] [PubMed] [Google Scholar]
- [30].Dwivedi S, Kar SK, Alcohol binge drinking in OCD: A compulsive ritualistic behaviour to counter magical thinking, Asian journal of psychiatry 29 (2017) 160–161. [DOI] [PubMed] [Google Scholar]
- [31].Geller DA, McGuire JF, Orr SP, Small BJ, Murphy TK, Trainor K, Porth R, Wilhelm S, Storch EA, Fear extinction learning as a predictor of response to cognitive behavioral therapy for pediatric obsessive compulsive disorder, Journal of anxiety disorders 64 (2019) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim YJ, Lim JA, Lee JY, Oh S, Kim SN, Kim DJ, Ha JE, Kwon JS, Choi JS, Impulsivity and compulsivity in Internet gaming disorder: A comparison with obsessive-compulsive disorder and alcohol use disorder, Journal of behavioral addictions 6(4) (2017) 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McLaughlin NC, Strong D, Abrantes A, Garnaat S, Cerny A, O’Connell C, Fadok R, Spofford C, Rasmussen SA, Milad MR, Greenberg BD, Extinction retention and fear renewal in a lifetime obsessive-compulsive disorder sample, Behavioural brain research 280 (2015) 72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cromwell HC, Berridge KC, Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax, The Journal of neuroscience : the official journal of the Society for Neuroscience 16(10) (1996) 3444–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Berridge KC, Aldridge JW, Houchard KR, Zhuang X, Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: a model of obsessive compulsive disorder and Tourette’s, BMC biology 3 (2005) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].John CS, Sypek EI, Carlezon WA, Cohen BM, Ongur D, Bechtholt AJ, Blockade of the GLT-1 Transporter in the Central Nucleus of the Amygdala Induces both Anxiety and Depressive-Like Symptoms, Neuropsychopharmacology 40(7) (2015) 1700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gasull-Camos J, Tarres-Gatius M, Artigas F, Castane A, Glial GLT-1 blockade in infralimbic cortex as a new strategy to evoke rapid antidepressant-like effects in rats, Transl Psychiatry 7(2) (2017) e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thomas SJ, Gonsalvez CJ, Johnstone SJ, Neural time course of threat-related attentional bias and interference in panic and obsessive-compulsive disorders, Biological Psychology 94(1) (2013) 116–129. [DOI] [PubMed] [Google Scholar]
- [39].Geller DA, McGuire JF, Orr SP, Pine DS, Britton JC, Small BJ, Murphy TK, Wilhelm S, Storch EA, Fear conditioning and extinction in pediatric obsessive-compulsive disorder, Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists 29(1) (2017) 17–26. [PMC free article] [PubMed] [Google Scholar]
- [40].McGuire JF, Orr SP, Wu MS, Lewin AB, Small BJ, Phares V, Murphy TK, Wilhelm S, Pine DS, Geller D, Storch EA, Fear Conditioning and Extinction in Youth with Obsessive-Compulsive Disorder, Depression and anxiety 33(3) (2016) 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lang M, Kratky J, Shaver JH, Jerotijevic D, Xygalatas D, Effects of Anxiety on Spontaneous Ritualized Behavior, Current biology : CB 25(14) (2015) 1892–7. [DOI] [PubMed] [Google Scholar]
- [42].Godar SC, Bortolato M, What makes you tic? Translational approaches to study the role of stress and contextual triggers in Tourette syndrome, Neuroscience and biobehavioral reviews 76(Pt A) (2017) 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jin X, Tecuapetla F, Costa RM, Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences, Nature Neuroscience 17(3) (2014) 423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, Janssen AL, Udvardi PT, Shiban E, Spilker C, Balschun D, Skryabin BV, Dieck S, Smalla KH, Montag D, Leblond CS, Faure P, Torquet N, Le Sourd AM, Toro R, Grabrucker AM, Shoichet SA, Schmitz D, Kreutz MR, Bourgeron T, Gundelfinger ED, Boeckers TM, Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2, Nature 486(7402) (2012) 256–60. [DOI] [PubMed] [Google Scholar]
- [45].Sugimoto J, Tanaka M, Sugiyama K, Ito Y, Aizawa H, Soma M, Shimizu T, Mitani A, Tanaka K, Region-specific deletions of the glutamate transporter GLT1 differentially affect seizure activity and neurodegeneration in mice, Glia 66(4) (2018) 777–788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.