Abstract
Objectives
We aimed at analyzing the serum levels of citrullinated histone H3 (CitH3) in patients with dermatomyositis (DM) and their association with disease activity.
Methods
Serum CitH3 levels were measured using enzyme‐linked immunosorbent assays in serum samples obtained from 93 DM patients and 56 healthy controls (HCs). Receiver operating characteristic (ROC) curve analysis was performed to evaluate the discriminant capacity of CitH3 and other disease variables. The association between CitH3 and disease variables was analyzed using Pearson's rank correlation.
Results
Serum CitH3 level was significantly lower in DM patients than in HCs (p < 0.001). The ROC curve analysis revealed that CitH3 strongly discriminated DM patients from HCs (area under the curve [AUC], 0.86), and a combination of CitH3 and the ratio of neutrophil to lymphocyte counts (NLR) showed a greater diagnostic value (AUC, 0.92). Serum CitH3 levels were markedly lower in DM patients with normal muscle enzyme levels than in HCs (all p < 0.001), and when compared to an elevated group, the CitH3 levels were comparable (all p > 0.05). The CitH3 levels showed no difference between DM in active and remission groups. However, in a paired test with 18 hospitalized DM patients, the CitH3 levels were higher in remission state than in active state. Moreover, the CitH3 levels showed no correlation with disease variables that were associated with the disease activity of DM.
Conclusions
Serum CitH3 level may serve as a useful biochemical marker for screening patients with DM from HCs, while its role in monitoring DM disease activity requires further research.
Keywords: biochemical marker, citrullinated histone H3, dermatomyositis, disease activity
In DM patients, the serum level of CitH3 was significantly lower when compared to HCs. ROC curve analysis revealed that CitH3 level strongly discriminated DM patients from HCs, and the combination of CitH3 with NLR had shown a greater diagnostic value. Furthermore, CitH3 level showed no difference in DM patients with active disease when compared to remission. However, in a small number paired test with 18 hospitalized DM patients, CitH3 levels were markedly higher in DM patients with remission compared to those with active. Hence, the role of CitH3 to discriminate DM patients in active or in remission needs further research.
1. INTRODUCTION
Idiopathic inflammatory myopathies (IIMs), including dermatomyositis (DM), polymyositis (PM), inclusion body myositis, and immune‐mediated necrotizing myopathy, are rare systemic autoimmune diseases. 1 , 2 The incidence of IIMs is fairly low, as reported by several research groups. 3 , 4 , 5 Among IIMs, DM is the most common. 6 Both children and adults may develop DM with an overall female‐to‐male ratio of approximately 2:1. 7 Patients with DM are characterized by chronic proximal muscle inflammation and weakness accompanied by characteristic skin rashes, such as periorbital purple‐red edema, Gottron sign, and nail fold rigid dilated capillary erythema. 6 At present, the diagnosis of DM is mainly based on symmetrical proximal muscle weakness, pain, and tenderness, accompanied by a characteristic skin rash, as mentioned above. 8 Increased serum enzyme activity is an important serological indicator in the diagnosis of this disease. 9 Muscle enzyme testing includes serum creatine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) activity and has been shown to moderately correlate with disease activity in IIMs. 9 , 10 However, the serum levels of muscle enzymes, generally used to evaluate muscle inflammation, may be in the normal range at the early stage of the disease, or even throughout the disease process, thereby making it more difficult to establish a definite diagnosis of DM. 10 , 11 Moreover, patients with DM may develop serum autoantibodies, such as antinuclear antibodies and myositis‐specific autoantibodies. 12 However, the positive rate of diagnosis is low, and early diagnosis is difficult. 9 Therefore, it is important to identify early novel diagnostic indicators.
The histological features of DM include infiltration of mononuclear and inflammatory cells in skeletal muscle tissue, leading to muscle damage and dysfunction by releasing cytokines, cytotoxic molecules, or proteinase. 9 In DM, most of the previous work has focused on the role of the adaptive immune system and lymphocyte subsets, and recent research on the cellular components of the innate immune system, such as neutrophils, is beginning to be investigated. 13 , 14 , 15 , 16 Neutrophils that form neutrophil extracellular traps (NETs) have recently been proposed to play important pathogenic roles in systemic autoimmune diseases, including IIMs. 14 , 15 Neutrophil extracellular traps and infiltrating neutrophils are present in IIM muscle tissues, and they enhance neutrophil gene signatures which associated with muscle injury. 15 Importantly, experiments revealed that IIM NETs decreased the viability of myotubes in a citrullinated histone‐dependent but native histone‐independent manner, which indicated the important roles of citrullinated histones in the development of IIM. 15
Citrullinated histones are products of the posttranslational conversion of peptidylarginine to citrulline of histones under the activation of the enzyme peptidylarginine deiminase. Citrullinated histone 3 (CitH3) is an important modified form of histone H3 that promotes chromatin decondensation during NET formation. 17 CitH3 can be detected in the nucleus of neutrophils upon stimulation, 17 but it is also released into the bloodstream upon NETosis. 18 Recent research has focused on elucidating the role of circulating CitH3 in diagnostics, its correlation with disease activity, and its prognostic relevance. 19 , 20 , 21 A study by Tian et al. 20 revealed that in patients with septic shock, serum level of CitH3 increased and correlated with disease severity and prognosis, which may yield vital insights into the pathophysiology of sepsis. Moreover, in patients with acute pancreatitis (AP), CitH3 concentration increased in septic AP and was closely correlated with disease severity and clinical outcome. 19 In addition to sepsis, in patients with advanced cancer, 22 pneumonia, 23 and coronavirus disease 2019 (COVID‐19), 24 the serum levels of CitH3 were also increased. However, the role of circulating CitH3 in the diagnosis of IIM remains unclear, although citrullinated histones may play an important role in IIM development. Considering that DM is the most common type of IIMs, this study aimed at evaluating serum CitH3 levels in patients with DM. We also focused on identifying the correlation between CitH3 and DM disease severity to advance further research on DM pathogenesis.
2. MATERIALS AND METHODS
2.1. Human participants
The study group comprised 95 DM patients treated at the Xiangya Hospital between May 2018 and December 2021. Patients with DM fulfilled the Bohan and Peter diagnostic criteria 25 , 26 and had no other systemic autoimmune diseases, infections, or other major illnesses. Disease activity was assessed using the Myositis Disease Activity Assessment (MYOACT) established by the International Myositis Assessment and Clinical Studies group. 27 This tool has proven to be reliable for evaluating disease activity in Chinese patients with DM or PM. 28 Active disease was defined by clinicians based on clinical criteria, including typical skin manifestations, muscle involvement, and/or an increase in muscle enzyme levels. In this study, the patients with DM also had arthritis (19.4%), dysphagia (3.2%), or ulceration (4.3%). Additionally, 58% of the patients with myositis were diagnosed with interstitial lung disease. Interstitial lung disease was identified using high‐resolution computed tomography. Note that the group of DM patients in the present study included a subset of patients published in our previous report. 16 The treatment status of the included patients with DM comprised a daily glucocorticoid dose alone or concomitant immunosuppressant use. For comparison, age‐ and sex‐matched individuals were also included as healthy controls (HCs, n = 56) recruited from adults who underwent routine physical examination and had no chronic medical problems or medications.
Written informed consent was obtained from all the patients, and the study was approved by the Ethics Committee of Xiangya Hospital, Central South University, where the study was performed.
2.2. Laboratory analysis
The following additional data for patients with IIMs were collected: CK, LDH, ALT, AST, erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP), and neutrophil and lymphocyte counts. These were all analyzed using routine laboratory techniques within 24 h of enrolment. Antinuclear antibody (ANA) was detected by indirect immunofluorescence using Hep‐2 cells (Euroimmun). Myositis‐specific and myositis‐associated autoantibodies were detected using immunoblotting (Euroimmun). The demographic data for the disease groups are shown in Table 1.
TABLE 1.
Clinical characteristics of the study subjects.
| DM | HC | p value | |
|---|---|---|---|
| Number | 95 | 56 | |
| Gender (female/male) | 73/22 | 49/7 | 0.108 |
| Age (years) | 50 (43–58.5) | 49.5 (43.5–57.25) | 0.923 |
| Disease durations (years) | |||
| Active/remission (number) | 74/39 a | ||
| MYOACT | 1.587 (1.031–2.104) | ||
| Muscle enzymes | |||
| CK, uKat/L | 59.7 (41.95–159.35) | ||
| AST, uKat/L | 36.7 (23.6–65.8) | 22.8 (19.5–26.3) | 0.000*** |
| ALT, uKat/L | 29.8 (18–64.9) | 19.3 (13.9–24.4) | 0.000*** |
| LDH, uKat/L | 299 (228–383.5) | ||
| Inflammation marker | |||
| CRP, mg/L | 3.8 (2.6–9.8) | ||
| ESR, mm/h | 43.5 (27.3–73.5) | ||
| Neutrophil, ×109/L | 4.6 (3.3–6.4) | 3.2 (2.7–3.9) | 0.000*** |
| Lymphocyte, ×109/L | 1.1 (0.7–1.5) | 1.8 (1.5–2.0) | 0.000*** |
| NLR | 4.3 (2.4–7.0) | 1.8 (1.4–2.6) | 0.000*** |
| PLT, ×109/L | 207.5 (161.5–262.8) | 216.5 (189.3–260.5) | 0.258 |
Note: Data are presented as number of patients or median (Q25–Q75). Differences between groups were assessed with the Mann–Whitney U test. A p value < 0.05 was used to indicate a statistically significant result (***p < 0.001).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine phosphokinase; CRP, C‐reactive protein; DM, dermatomyositis; ESR, erythrocyte sedimentation rate; HC, healthy controls; LDH, lactate dehydrogenase; MYOACT, myositis disease activity assessment; NLR, the ratio of neutrophil to lymphocyte counts; PLT, platelet.
Among 39 remission samples, 18 of them are paired samples.
2.3. Measurement of serum CitH3 levels
Serum samples (1 mL) were collected in Eppendorf tubes and stored at −20°C until analysis. Human CitH3 serum levels were measured using a commercially available enzyme‐linked immunosorbent assay (ELISA) kit (Cayman Chemicals, Inc.). The ELISA kit instructions showed that the minimum detection level of CitH3 was 0.1 ng/mL, and the inter‐ and intra‐assay coefficients of variations at 1.25 ng/mL were 13.2% and 7.2%, respectively. Standards and patient samples were analyzed in duplicate, according to the manufacturer's instructions.
2.4. Statistical analysis
Data were tested for normality using the Kolmogorov–Smirnov test. Paired data collected in the experiment were compared using a paired‐sample t test. Differences between unpaired groups were assessed using the Mann–Whitney U test. Data are shown as the mean ± standard deviation or median (interquartile range). Correlations between variables were evaluated using the Pearson's rank correlation. Receiver operating characteristic (ROC) curves were used to evaluate the significance of CitH3 levels and other disease variables in distinguishing patients with DM and HCs. The Youden index was calculated as a sensitivity + specificity − 1. The best critical point (cutoff value) was selected as the largest tangential point of the Youden index. Statistical significance was set at p < 0.05. Statistical analysis was performed using SPSS 20.0 (IBM SPSS Statistics, IBM Corporation) or Prism software (version 5.0; GraphPad Software, Inc.).
3. RESULTS
3.1. Serum levels of CitH3 in patients with DM and controls
The concentrations of CitH3 in the patients with DM and HCs are shown in Figure 1A. Serum CitH3 levels in the DM patients were particularly lower than those in the HCs (6.6 [3.7–12.0] vs. 33.6 [18.5–45.3] ng/mL, median [interquartile range]; p < 0.001; Figure 1A). The predictive value of CitH3 in patients with DM versus HCs was studied using a univariate ROC analysis. The univariate areas under the curve (AUC) of CitH3 were 0.86 (95% CI: 0.81–0.94), sensitivity of 0.78, and specificity was 0.839 for discriminating between patients with DM and HCs (Figure 1B and Table 2).
FIGURE 1.
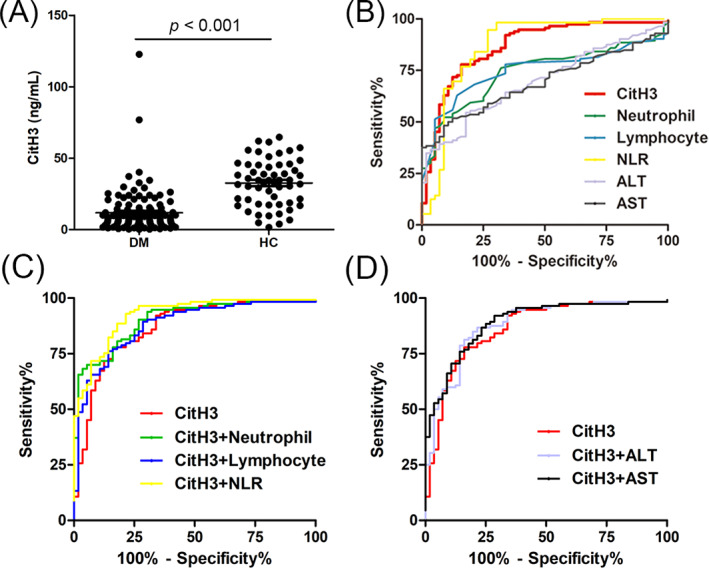
Serum levels of citrullinated histone H3 (CitH3) in patients with dermatomyositis (DM). (A) CitH3 levels in DM patients and healthy controls (HCs). (B) The predictive values of CitH3 (area under the curve [AUC], 0.86), neutrophil (AUC 0.74), lymphocyte (AUC 0.75), the ratio of neutrophil to lymphocyte counts (NLR, AUC 0.87), alanine aminotransferase (ALT, AUC 0.70), and aspartate aminotransferase (AST, AUC 0.69) to distinguish DM and HCs. (C) The predictive values of the combination of CitH3 with neutrophil (AUC 0.90), lymphocyte (AUC 0.88), or NLR (AUC 0.92) to distinguish DM and HCs. (D) The predictive values of the combination of CitH3 with ALT (AUC 0.88) or AST (AUC 0.89) to distinguish DM and HCs. Each dot represents the CitH3 level of each individual. The horizontal lines represent the mean levels of CitH3 in each group. p values were calculated by Mann–Whitney U test, and a p value < 0.05 was used to indicate a statistically significant result.
TABLE 2.
AUC, sensitivity and specificity for CitH3, other serological markers and combinations.
| AUC (95% CI) | Cut off value | Sensitivity (%) | Specificity (%) | p value | |
|---|---|---|---|---|---|
| CitH3 | 0.86 (0.80–0.93) | 16.095 ng/mL | 78.0 | 83.9 | <0.001*** |
| Neutrophil | 0.74 (0.67–0.82) | 3.25 × 109/L | 76.1 | 67.9 | <0.001*** |
| Lymphocyte | 0.75 (0.67–0.82) | 1.35 × 109/L | 62.8 | 85.7 | <0.001*** |
| NLR | 0.87 (0.80–0.94) | 2.75 | 68.1 | 91.1 | <0.001*** |
| ALT | 0.70 (0.62–0.78) | 25.8 uKat/L | 54.5 | 82.1 | <0.001*** |
| AST | 0.69 (0.61–0.77) | 29.0 uKat/L | 51.8 | 87.5 | <0.001*** |
| CitH3+Neutrophil | 0.90 (0.86–0.95) | 0.22 | 68.1 | 96.4 | <0.001*** |
| CitH3+Lymphocyte | 0.88 (0.82–0.93) | 0.28 | 76.1 | 85.7 | <0.001*** |
| CitH3+NLR | 0.92 (0.88–0.96) | 0.49 | 92.9 | 78.6 | <0.001*** |
| CitH3+ALT | 0.88 (0.83–0.94) | 0.37 | 86.6 | 78.6 | <0.001*** |
| CitH3+AST | 0.89 (0.84–0.94) | 0.35 | 86.6 | 76.8 | <0.001*** |
Note: A p value < 0.05 was used to indicate a statistically significant result (***p < 0.001).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve; CitH3, citrullinated histone H3; NLR, the ratio of neutrophil to lymphocyte counts; 95% CI, 95% confidence interval.
Considering the diagnostic values of muscle enzymes and inflammatory markers in patients with DM (Figure 1B and Table 2), we further tested the predictive value of the combination of CitH3 and the abovementioned markers. The results showed that a combination of CitH3 with NLR had the greatest diagnostic value for distinguishing DM from HCs, with an AUC of 0.92 (0.88–0.96), sensitivity of 0.93, and specificity of 0.79 (Figure 1C and Table 2).
3.2. Serum levels of CitH3 in normal muscle enzymes group
Muscle enzyme activity is an important serological indicator for the diagnosis of DM, but the levels of muscle enzymes in nearly half of DM patients are not elevated even throughout the disease process. Hence, we further compared the serum CitH3 levels in normal muscle enzyme group of the patients with DM and HC. As shown in Figure 2A, the serum CitH3 levels in a CK normal DM group (0–200 uK/L, n = 74) were markedly lower than those in the HCs (p < 0.001), while there was no difference in the CK elevated group (>200 uK/L, n = 21) (p = 0.473). Similar results were observed in an LDH normal DM group (0–300 uK/L, n = 49) versus HCs, ALT normal DM group (0–40 uK/L, n = 59) versus HCs, and AST normal DM group (0–40 uK/L, n = 59) versus HCs (both p < 0.001). Moreover, the serum CitH3 levels showed no differences between the LDH normal group and LDH elevated group (>300 uK/L, n = 46; p = 0.150), ALT normal group versus ALT elevated group (>40 uK/L, n = 35; p = 0.257), and AST normal group versus AST elevated group (>40 uK/L, n = 35; p = 0.111).
FIGURE 2.
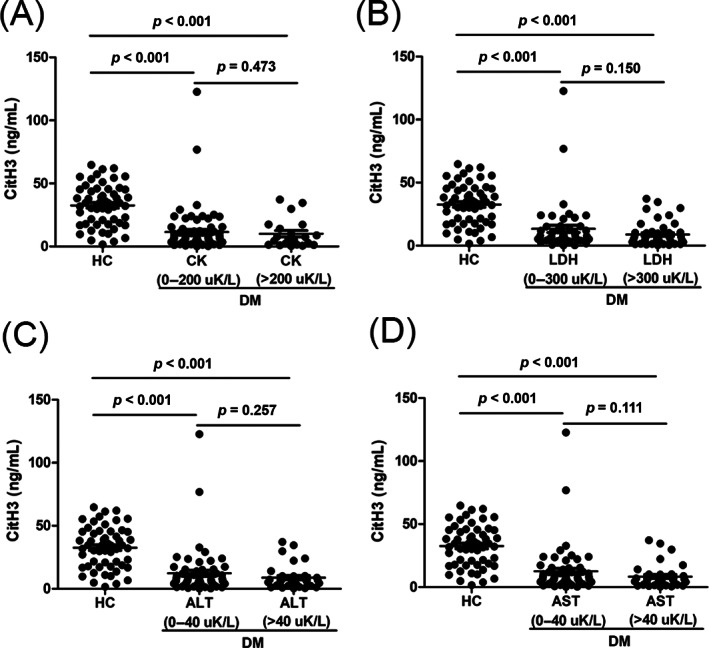
Serum levels of citrullinated histone H3 (CitH3) in normal muscle enzymes group. (A) The serum CitH3 levels in creatine phosphokinase (CK) normal dermatomyositis (DM) group (0–200 uK/L), CK elevated DM group (>200 uK/L), and healthy controls (HCs). (B) The serum CitH3 levels in lactate dehydrogenase (LDH) normal DM group (0–300 uK/L), LDH elevated DM group (>300 uK/L) and HCs. (C) The serum CitH3 levels in alanine aminotransferase (ALT) normal DM group (0–40 uK/L), ALT elevated DM group (>40 uK/L), and HCs. (D) The serum CitH3 levels in aspartate aminotransferase (AST) normal DM group (0–40 uK/L), AST elevated DM group (>40 uK/L), and HCs. Each dot represents the CitH3 level of each individual. The horizontal lines represent the mean levels of CitH3 in each group. p values were calculated using Mann–Whitney U test, and p < 0.05 was used to indicate a statistically significant result.
3.3. Correlation of CitH3 with disease activity
To investigate the correlation between CitH3 levels and disease activity, we initially compared the DM patients in active with those in remission. Results showed that the serum CitH3 levels were comparable in the DM patients with active disease and those in remission (6.3 [3.7–10.9] vs. 8.7 [3.9–14.0], p = 0.479; Figure 3A).
FIGURE 3.
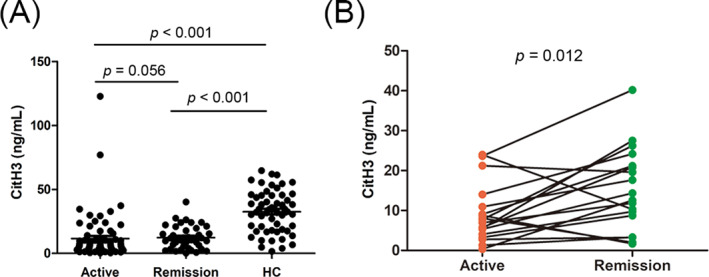
Serum levels of citrullinated histone H3 (CitH3) in dermatomyositis (DM) patients with active disease and remission. (A) The level of CitH3 in DM patients with active disease and remission. (B) The level of CitH3 in paired active and remission DM patients (n = 18). Each dot represents the CitH3 level of each individual. The horizontal lines represent the mean levels of CitH3 in each group. p values were calculated using Mann–Whitney U and paired‐sample t tests. A p value of <0.05 was used to indicate a statistically significant result.
To further explore the role of CitH3 in distinguishing between patients with active DM and those in remission, paired sera from 18 hospitalized patients in a state of active and remission were collected and tested. The CitH3 levels were markedly higher in the remission state of the DM patients when compared to in active state (15.29 ± 10.36 vs. 9.04 ± 7.24, p = 0.012; Figure 3B).
Correlation analysis revealed no correlations between serum CitH3 levels and MYOACT (which represents the disease activity of DM), LDH, CK, AST, and ALT. Moreover, serum CitH3 levels showed no correlation with inflammatory markers, such as CRP, ESR, neutrophils, and lymphocytes. The associated data are presented in Table 3.
TABLE 3.
Association of CitH3 levels with laboratory parameters of patients with DM.
| CitH3 | p value | |
|---|---|---|
| MYOACT | 0.047 | 0.653 |
| Muscle enzymes | ||
| CK | −0.04 | 0.647 |
| LDH | −0.08 | 0.386 |
| ALT | −0.16 | 0.085 |
| AST | −0.13 | 0.156 |
| Inflammation marker | ||
| CRP | −0.05 | 0.613 |
| ESR | 0.05 | 0.653 |
| Neutrophil | −0.08 | 0.389 |
| Lymphocyte | −0.02 | 0.836 |
| NLR | −0.09 | 0.322 |
Note: Data were analyzed using the Pearson's rank correlation. A p value < 0.05 was used to indicate a statistically significant result.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CitH3, citrullinated histone H3; CK, creatine phosphokinase; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; MYOACT, the myositis disease activity assessment; NLR, the ratio of neutrophil to lymphocyte counts.
3.4. Association of CitH3 with autoantibodies in patients with DM
In patients with DM, the prevalence of ANA, anti‐Ro‐52, anti‐MDA5, anti‐TIF‐1γ, anti‐Mi‐2, and anti‐PM‐Scl was 83.9%, 46.5%, 39.5%, 20.9%, 15.1%, and 12.8%, respectively. The prevalence of other autoantibodies was all less than 10%. We examined the differences in serum levels of CitH3 in DM patients with and without elevation of an autoantibody. Our data showed that the serum levels of CitH3 were higher in the anti‐TIF‐1γ‐positive group than in the anti‐TIF‐1γ‐negative group (13.83 ± 9.13 vs. 8.32 ± 8.23, ng/mL, p = 0.016) in the DM patients. In contrast, no significant differences were observed between other autoantibody‐positive and autoantibody‐negative groups. The prevalence of autoantibodies and association between CitH3 and autoantibodies are summarized in Table 4.
TABLE 4.
Associations of CitH3 with autoantibodies in DM patients.
| N (Pos/Neg) | CitH3 | |||
|---|---|---|---|---|
| Pos | Neg | p value | ||
| ANA | 74/15 | 11.81 ± 17.55 | 5.62 ± 4.05 | 0.180 |
| Anti‐Ro‐52 | 40/48 | 9.38 ± 9.30 | 9.48 ± 8.02 | 0.956 |
| Anti‐MDA5 | 34/54 | 10.42 ± 9.44 | 10.42 ± 9.44 | 0.173 |
| Anti‐TIF‐1γ | 19/69 | 13.51 ± 8.98 | 8.31 ± 8.17 | 0.018* |
| Anti‐Mi‐2 | 14/74 | 9.95 ± 9.20 | 9.34 ± 8.51 | 0.806 |
| Anti‐PM‐Scl | 11/77 | 9.17 ± 8.33 | 9.17 ± 8.33 | 0.446 |
| Anti‐Jo‐1 | 8/80 | 9.98 ± 13.10 | 9.38 ± 8.11 | 0.851 |
| Anti‐NXP2 | 6/82 | 12.75 ± 12.54 | 9.19 ± 8.27 | 0.330 |
| Anti‐SAE1 | 5/83 | 7.99 ± 2.69 | 9.52 ± 8.80 | 0.701 |
| Anti‐PL7 | 4/84 | 11.66 ± 12.37 | 9.33 ± 8.44 | 0.597 |
| Anti‐EJ | 3/85 | 10.54 ± 11.95 | 9.39 ± 8.53 | 0.821 |
| Anti‐SRP | 2/86 | 5.40 ± 5.24 | 9.53 ± 8.63 | 0.504 |
| Anti‐Ku | 1/87 | / | / | / |
Note: Data are presented as number of patients or mean ± standard deviation. Differences between groups were assessed with the Mann–Whitney U test. A p value < 0.05 was used to indicate a statistically significant result (*p < 0.05).
Abbreviations: ANA, antinuclear antibody; Anti‐EJ, anti‐glycyl transfer RNA antibodies; Anti‐Jo‐1, anti‐histidyl transfer RNA antibody; Anti‐Ku, antibodies to Ku; anti‐MDA5, antimelanoma differentiation‐associated gene 5 antibodies; Anti‐Mi‐2, antibodies to Mi‐2; Anti‐NXP2, antinuclear matrix protein 2 antibody; Anti‐PL7, anti‐threonyl transfer RNA antibody; Anti‐PM‐Scl, anti‐polymyositis/scleroderma antibodies; anti‐Ro‐52, antibodies to Ro‐52; Anti‐SAE1, anti‐small ubiquitin‐like modifier activating enzyme 1 antibody; Anti‐SRP, anti‐signal recognition particle antibody; anti‐TIF‐1γ, antitranscriptional intermediary factor 1 antibody.
4. DISCUSSION
In this study, the CitH3 levels were significantly lower in the DM patients than in the HCs. The ROC curve analysis revealed that the CitH3 level strongly discriminated the DM patients from the HCs (AUC 0.86), even in the normal muscle enzymes DM group. This study is the first to focus on CitH3 as a serological biomarker to distinguish DM patients from HCs.
Extracellular histones in serum are mainly derived from NETs produced by activated neutrophils. 29 CitH3 is the product of posttranslational conversion of peptidylarginine to citrulline at the N terminus of histone H3. Levels of circulating CitH3 have been reported to be significantly increased in patients with septic shock, 20 septic AP, 19 advanced cancer, 22 pneumonia, 23 and coronavirus disease 2019 (COVID‐19). 24 Different from the abovementioned, our results showed that the serum level of CitH3 is markedly decreased in DM patients when compared to HCs. A similar decline in CitH3 levels was observed in oral squamous cell carcinoma (OSCC) patients, and the expression of CitH3 was statistically and significantly lower in the neutrophils of OSCC patients than in the control group. 30 A decline in CitH3 in OSCC patients was at the cellular level, which indicates that the process of NETosis in OSCC is disrupted. 30 Zhang et al. 14 reported that in DM/PM patients, excessively formed NETs cannot be completely degraded because of decreased DNase I activity, suggesting that abnormal regulation of NETs may be involved in DM/PM, which may lead to a decrease in CitH3 in the serum. In this study, we only tested the serum level of CitH3 in the DM patients; the cellular level of CitH3 in the neutrophils of DM patients remains unknown and requires further investigation. Moreover, the CitH3 level in pathological muscle tissue of DM also requires further investigation, and research has revealed that IIM NETs decreases the viability of myotubes in a citrullinated histone‐dependent manner, indicating the important role of citrullinated histones in IIM. 15
Consistent with our results, several studies have reported that muscle enzyme activity, 10 neutrophil count, and NLR 31 , 32 are serological indicators that distinguish patients with DM from HCs. In this study, we focused on CitH3 as a serological biomarker for DM diagnosis. The combination of CitH3 with the abovementioned markers showed superior diagnostic value, particularly the combination of CitH3 with NLR. Further, our research showed that in the DM patients with normal muscle enzymes (including CK, LDH, ALT, and AST), serum CitH3 levels were markedly lower than those in HCs, and the serum CitH3 levels between the normal muscle enzyme group and elevated group were comparable. This means that even if the levels of the characteristic muscle enzymes of DM are normal, we can distinguish DM from HCs by the serum level of CitH3.
The discovery of new biomarkers for monitoring disease activity in patients with IIM remains a topic of great interest. Previous studies have revealed that the serum level of CitH3 is closely correlated with disease severity in patients with septic shock 20 and septic AP. 19 Moreover, Thalin et al. 22 found that high levels of circulating CitH3 strongly predicted poor clinical outcomes in a cohort of cancer patients with a twofold increased the risk of short‐term mortality. However, further research showed that the level of CitH3 may have no correlation or at least a weak correlation with the disease activity of DM. This conclusion is based on two aspects of the data. First, in patients with active and remission disease, the levels of CitH3 were comparable. Further paired test of 18 hospitalized patients revealed that CitH3 levels in DM with an active state were significantly lower than DM in remission state, among four patients showed an upward trend. Second, serum CitH3 levels showed no correlation with disease activity‐associated factors (MYOACT, CK, ALT, AST, and LDH) or inflammation markers (CRP, ESR, neutrophil, lymphocyte, and NLR). These results indicate that a larger sample size of the paired test is needed to further carry out this experiment.
Despite the novel and clinically relevant findings in this study, there are some limitations. The patients included in our study differed in terms of disease severity and treatment options. In order to eliminate the influence of abovementioned factors, larger sample size of the paired test was needed to confirm the correlation of serum levels of CitH3 with the disease activity of DM. Additionally, in this study, we only tested the serum level of CitH3 in DM patients; the cellular level of CitH3 in the neutrophils of DM patients and the CitH3 level in pathological muscle tissue remain unknown. Thus, further research on CitH3 is warranted in future studies.
CONFLICT OF INTEREST STATEMENT
None.
ACKNOWLEDGMENTS
The study was supported by the Natural Science Foundation of Hunan Province of China (No. 2021JJ41029).
Wang W, Peng W, Wu S. Low serum level of citrullinated histone H3 in patients with dermatomyositis. J Clin Lab Anal. 2023;37:e24876. doi: 10.1002/jcla.24876
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article. If any additional information is required it may be obtained by request with the corresponding author.
REFERENCES
- 1. Dalakas MC, Sivakumar K. The immunopathologic and inflammatory differences between dermatomyositis, polymyositis and sporadic inclusion body myositis. Curr Opin Neurol. 1996;9(3):235‐239. [DOI] [PubMed] [Google Scholar]
- 2. Luo YB, Mastaglia FL. Dermatomyositis, polymyositis and immune‐mediated necrotising myopathies. Biochim Biophys Acta. 2015;1852(4):622‐632. [DOI] [PubMed] [Google Scholar]
- 3. Smoyer‐Tomic KE, Amato AA, Fernandes AW. Incidence and prevalence of idiopathic inflammatory myopathies among commercially insured, Medicare supplemental insured, and Medicaid enrolled populations: an administrative claims analysis. BMC Musculoskelet Disord. 2012;13:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohta A, Nagai M, Nishina M, Tomimitsu H, Kohsaka H. Prevalence and incidence of polymyositis and dermatomyositis in Japan. Mod Rheumatol. 2014;24(3):477‐480. [DOI] [PubMed] [Google Scholar]
- 5. Cho SK, Kim H, Myung J, et al. Incidence and prevalence of idiopathic inflammatory myopathies in Korea: a nationwide population‐based study. J Korean Med Sci. 2019;34(8):e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971‐982. [DOI] [PubMed] [Google Scholar]
- 7. Jakubaszek M, Kwiatkowska B, Maslinska M. Polymyositis and dermatomyositis as a risk of developing cancer. Reumatologia. 2015;53(2):101‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aggarwal R, Rider LG, Ruperto N, et al. 2016 American College of Rheumatology/European League Against Rheumatism criteria for minimal, moderate, and major clinical response in adult dermatomyositis and polymyositis: an International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2017;69(5):898‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang SH, Chang C, Lian ZX. Polymyositis and dermatomyositis–challenges in diagnosis and management. J Transl Autoimmun. 2019;2:100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Volochayev R, Csako G, Wesley R, Rider LG, Miller FW. Laboratory test abnormalities are common in polymyositis and dermatomyositis and differ among clinical and demographic groups. Open Rheumatol J. 2012;6:54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pachman LM, Abbott K, Sinacore JM, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr. 2006;148(2):247‐253. [DOI] [PubMed] [Google Scholar]
- 12. Huang HL, Lin WC, Lin PY, Weng MY, Sun YT. The significance of myositis autoantibodies in idiopathic inflammatory myopathy concomitant with interstitial lung disease. Neurol Sci. 2021;42(7):2855‐2864. [DOI] [PubMed] [Google Scholar]
- 13. Ha YJ, Hur J, Go DJ, et al. Baseline peripheral blood neutrophil‐to‐lymphocyte ratio could predict survival in patients with adult polymyositis and dermatomyositis: a retrospective observational study. PLoS One. 2018;13(1):e0190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang S, Shu X, Tian X, Chen F, Lu X, Wang G. Enhanced formation and impaired degradation of neutrophil extracellular traps in dermatomyositis and polymyositis: a potential contributor to interstitial lung disease complications. Clin Exp Immunol. 2014;177(1):134‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seto N, Torres‐Ruiz JJ, Carmona‐Rivera C, et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight. 2020;5(3):e134189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu S, Peng W, Zhang Y, Guo J, Fu J, Wang W. Correlation of PMN elastase and PMN elastase‐to‐neutrophil ratio with disease activity in patients with myositis. J Transl Med. 2019;17(1):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Li M, Stadler S, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun. 2009;1(3):194‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan B, Li Y, Liu Y, Wang W, Huang G, Ouyang Y. Circulating CitH3 is a reliable diagnostic and prognostic biomarker of septic patients in acute pancreatitis. Front Immunol. 2021;12:766391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tian Y, Russo RM, Li Y, et al. Serum citrullinated histone H3 concentrations differentiate patients with septic verses non‐septic shock and correlate with disease severity. Infection. 2021;49(1):83‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan B, Alam HB, Chong W, et al. CitH3: a reliable blood biomarker for diagnosis and treatment of endotoxic shock. Sci Rep. 2017;7(1):8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thalin C, Lundstrom S, Seignez C, et al. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One. 2018;13(1):e0191231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Claushuis TAM, van der Donk LEH, Luitse AL, et al. Role of peptidylarginine deiminase 4 in neutrophil extracellular trap formation and host defense during Klebsiella pneumoniae‐induced pneumonia‐derived sepsis. J Immunol. 2018;201(4):1241‐1252. [DOI] [PubMed] [Google Scholar]
- 24. Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID‐19. JCI Insight. 2020;5(11):e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292(7):344‐347. [DOI] [PubMed] [Google Scholar]
- 26. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292(8):403‐407. [DOI] [PubMed] [Google Scholar]
- 27. Isenberg DA, Allen E, Farewell V, et al. International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult onset disease. Rheumatology (Oxford). 2004;43(1):49‐54. [DOI] [PubMed] [Google Scholar]
- 28. Shu XM, Lu X, Xie Y, Wang GC. Clinical characteristics and favorable long‐term outcomes for patients with idiopathic inflammatory myopathies: a retrospective single center study in China. BMC Neurol. 2011;11:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allam R, Kumar SV, Darisipudi MN, Anders HJ. Extracellular histones in tissue injury and inflammation. J Mol Med (Berl). 2014;92(5):465‐472. [DOI] [PubMed] [Google Scholar]
- 30. Garley M, Dziemianczyk‐Pakiela D, Ratajczak‐Wrona W, et al. NETs biomarkers in saliva and serum OSCC patients: one hypothesis, two conclusions. Adv Med Sci. 2022;67(1):45‐54. [DOI] [PubMed] [Google Scholar]
- 31. Gao MZ, Huang YL, Wu XD, et al. Red blood cell distribution width and neutrophil to lymphocyte ratio are correlated with disease activity of dermatomyositis and polymyositis. J Clin Lab Anal. 2018;32(1):e22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang W, Wang X, Zhang W, et al. Neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio are 2 new inflammatory markers associated with pulmonary involvement and disease activity in patients with dermatomyositis. Clin Chim Acta. 2017;465:11‐16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article. If any additional information is required it may be obtained by request with the corresponding author.


