Abstract
Nucleic acid therapeutics are promising alternatives to conventional anti-cancer therapy, such as chemotherapy and radiation therapy. While conventional therapies have limitations, such as high side effects, low specificity, and drug resistance, nucleic acid therapeutics work at the gene level to eliminate the cause of the disease. Nucleic acid therapeutics treat diseases in various forms and using different mechanisms, including plasmid DNA (pDNA), small interfering RNA (siRNA), anti-microRNA (anti-miR), microRNA mimics (miRNA mimic), messenger RNA (mRNA), aptamer, catalytic nucleic acid (CNA), and CRISPR cas9 guide RNA (gRNA). In addition, nucleic acids have many advantages as nanomaterials, such as high biocompatibility, design flexibility, low immunogenicity, small size, relatively low price, and easy functionalization. Nucleic acid therapeutics can have a high therapeutic effect by being used in combination with various nucleic acid nanostructures, inorganic nanoparticles, lipid nanoparticles (LNPs), etc. to overcome low physiological stability and cell internalization efficiency. The field of nucleic acid therapeutics has advanced remarkably in recent decades, and as more and more nucleic acid therapeutics have been approved, they have already demonstrated their potential to treat diseases, including cancer. This review paper introduces the current status and recent advances in nucleic acid therapy for anti-cancer treatment and discusses the tasks and prospects ahead.
Keywords: nucleic acid therapeutics (NATs), anti-cancer therapy, nucleic acid delivery, gene therapy, aptamer, RNA interference (RNAi)
1. Introduction
Cancer is a disease in which genetic defects occur due to various causes, such as carcinogens, viruses, and heredity, resulting in abnormal cell proliferation. Despite decades of research, cancer still remains a great threat to human health today [1,2]. Conventional anti-cancer treatments, such as radiation therapy, surgery, and chemotherapy, have limitations, such as strong side effects due to lack of specificity and drug resistance [3,4]. In contrast, gene therapy that can correct gene defects is a promising anti-cancer therapy with a specific, continuous therapeutic effect and low cytotoxicity and immunogenicity to normal cells [4,5,6]. Even now, gene therapy strategies continue to be developed and advanced, and regulating disease-related genes by utilization of nucleic acid therapeutics (NATs) is one of the approaches to achieving effective gene therapy [7,8].
1.1. Overview of Nucleic Acid Therapeutics (NATs)
Nitrogen bases, which are one of the main components of nucleic acids, form hydrogen bonds between complementary bases that are called base pairs. Through interstrand base pairing, they can hybridize into double-stranded structures, and through intrastrand base pairing, they can fold to form secondary structures. This is the most basic working principle of all NATs, which will be described below. NATs are nucleic acid strands that range in length from as short as 8–50 nucleotides to as long as several thousand or more nucleotides and operate through a variety of mechanisms, including regulating gene expression or protein activity. NATs include various forms of DNA and RNA, including plasmid DNA (pDNA), small interfering RNA (siRNA), anti-microRNA (anti-miR), microRNA (miR) mimics, antisense oligonucleotide (ASO), messenger RNA (mRNA), aptamer, catalytic nucleic acid (CNA), and CRISPR cas9 guide RNA (gRNA). NATs have the potential to achieve precision medicine and long-term treatment because they _target disease-related genes or proteins after being delivered intracellularly [9]. However, the application of NATs requires additional delivery strategies due to their low physiological stability owing to the presence of nucleases and low permeability to cell membranes due to a negatively charged phosphate backbone of nucleic acid [10,11]. These are part of the body’s defense mechanism against invasion of exogenous nucleic acid materials, and sometimes they may induce innate immune responses and lead to serious off-_target effects. To meet these requirements, various viral vector-based and nonviral-based delivery systems, such as DNA nanostructures (DNs), inorganic nanoparticles (INPs), and lipid nanoparticles (LNPs), have been developed that can protect NATs from degradation, isolate them from the external environment, increase cell membrane permeability, and maximize delivery to _target cells.
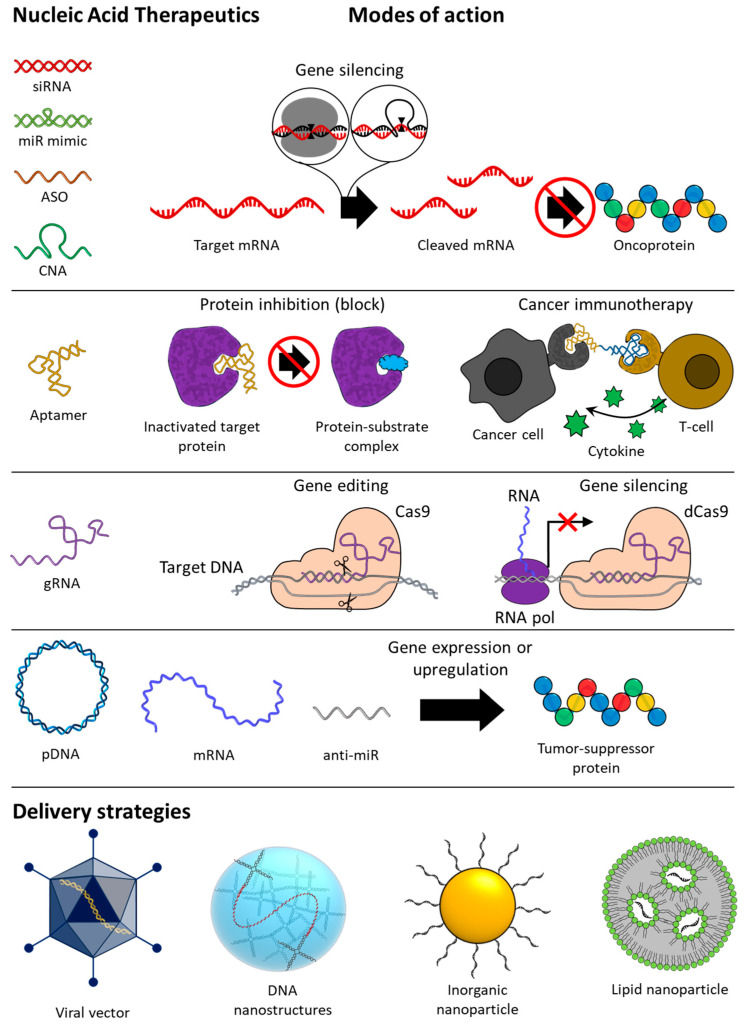
In recent decades, NATs have developed remarkably along with delivery technologies, and in this review, we would like to introduce the current status and recent advances of not only NATs but also the delivery technologies for anti-cancer treatment and discuss the tasks and prospects that lie ahead. The schematic image in Figure 1 summarizes the contents to be introduced in this review.
Figure 1.
Overview of types, modes of action, and delivery strategies of nucleic acid-based therapeutics for anti-cancer therapy. siRNA, small interfering RNA; miR, microRNA; ASO, antisense oligonucleotide; CNA, catalytic nucleic acid; gRNA, guide RNA; pDNA, plasmid DNA; mRNA, messenger RNA.
1.2. Nucleic Acid Therapeutics (NATs)
Since nucleic acids play a role in storing and transmitting genetic information within living organisms, the concept of regulating the expression of genes through the involvement of exogenous nucleic acid molecules has been around since the 1970s [12]. In the 1980s, the presence of ASO in prokaryotic cells revealed that nucleic acid-mediated gene regulation is a naturally occurring mechanism within cells, which gave credence to the development of NATs [13]. Several years later, the discovery that ribozymes, which recognize and cleave RNA at specific base sequences, act as effective gene regulators provided a major advance to the study of NATs as therapeutics [14]. Since then, research on NATs has made a lot of progress, and various forms of DNA and RNA therapeutics to be described below have been developed. NATs are largely divided into four modes of action: gene silencing, protein inhibition, gene editing, and gene expression (Figure 1). Although in vivo application of NATs requires additional intracellular delivery systems, the following sections describe NATs and delivery systems separately. First, we describe the mechanism of action and _target genes of each NAT, and then we describe four NAT delivery systems: viral vectors, DNs, INPs, and LNPs.
2. Gene Silencing
Gene silencing is a negative feedback mechanism to temporarily suppress the expression of specific genes by _targeting mRNA without adding or editing genes [15]. The treatment of cancer through gene silencing aims to reduce the expression levels of genes associated with the proliferation of cancer, such as anti-apoptosis and angiogenesis. This mechanism involves the process by which NATs recognize, hybridize, and cleavage _target mRNAs with complementary sequences.
2.1. RNA Interference (RNAi)
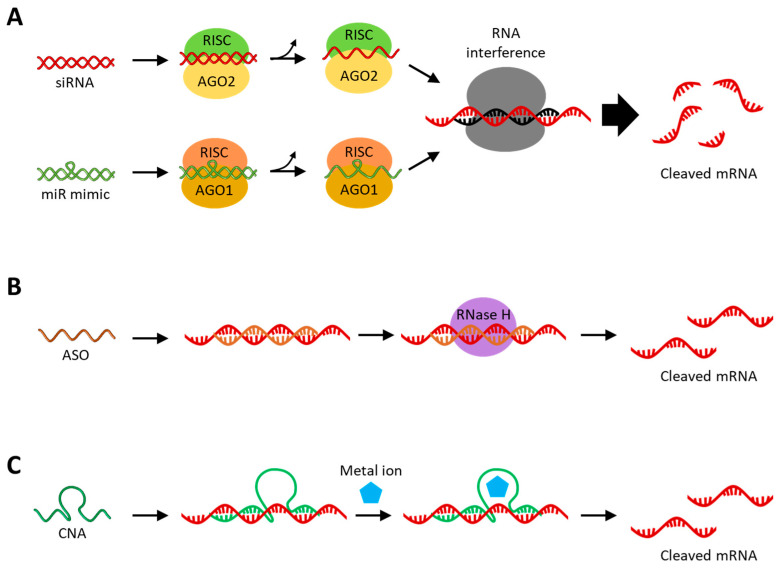
RNAi is an intracellular negative feedback process induced by double-stranded RNA, causing the degradation of specific mRNA _targets, which are defense mechanisms against external nucleic acid invasion [16,17]. siRNAs and miRNAs are non-coding RNAs that mediate RNAi and have received great attention for their high potential as therapeutics for various diseases [18]. As shown in Figure 2A, both siRNA and miRNA have RNAi activity in combination with the Argonaute protein (Ago), a component of the RNA-induced silencing complex (RISC). Although siRNA and miRNA have similar functions involved in RNAi in cytoplasm, they are of different origins. The differences between siRNA and miRNA are that siRNA is an externally derived exogenous RNA, while miRNA is an endogenous RNA derived from the genome. Additionally, siRNA inhibits a perfectly complementary mRNA, resulting in less off-_target effects, while miRNA inhibits multiple mRNAs that are partially complementary to the seed sequence [7,18,19]. Naturally occurring siRNAs are derived from exogenous long dsRNAs, and long dsRNAs are cleaved in the cytoplasm by the riboendonuclease Dicer to produce siRNAs with 21 to 23 base pairs [20]. After double-stranded siRNA is loaded onto Ago2 within the RISC, one strand is selected as the guide RNA, and the other strand, the passenger RNA, is cleaved and released. In this process, the RNA strand loaded onto the Ago2 is selected based on the difference in thermodynamic stability at the ends of the two small RNA strands [21]. Synthetic siRNAs can be treated as single-stranded or double-stranded; single-stranded siRNA is unstable and thus has a relatively low RNAi effect but exhibits RNAi activity quickly, whereas double-stranded siRNA exhibits RNAi activity relatively late but exhibits a longer RNAi effect with higher efficiency [22]. Although the double-stranded siRNA has higher physiological stability, there is also a concern that off-_target effects may occur, in which mRNA expression is suppressed by the passenger strand [23]. The approaches to anti-cancer therapy with synthetic siRNA can be carried out by internalizing synthetic siRNA into the _target cancer cells, thereby silencing the _target mRNA involved in cancer. These siRNA-_target genes are mainly those that promote cell differentiation and proliferation and inhibit apoptosis, which are overexpressed in cancer cells, such as Bcl-2 [24], survivin [25], epidermal growth factor receptor (EGFR) [26], Janus kinase 1 (JAK1) [27], and polo-like kinase 1 (PLK1) [28]. In addition, the JIP1 and CLN3 genes involved in resistance to chemo drugs and the PD-L1 and CTLA-4 genes acting on immune avoidance can also be _targeted by siRNA for anti-cancer therapy [29,30,31].
Figure 2.
(A) Schematic image of gene silencing with siRNA or miR mimic. The siRNA and miR mimic are incorporated within the RISC in the cytoplasm and then unwind, activating the guide strands. (B) Schematic image of gene silencing with RNase H-mediated cleavage with ASO. ASOs with chemically modified sugar backbones inhibit mRNA expression through steric hindrance. (C) Schematic image of gene silencing with CNA-based cleavage. The presence of metal ions helps to stabilize the activated structure of ribozymes and is essential for the catalytic activity of DNAzymes.
miRNAs are about 22 nt long RNAs that are synthesized through multiple processing steps in various pathways within living cells [32]. In the major synthesis pathway, miRNAs are first transcribed by RNA polymerase II (RNAP II) from genome to primary miRNA (pri-miRNA) with a double-stranded hairpin-loop structure, typically over 1 kb long. Afterwards, the pri-miRNA undergoes a post-transcriptional modification process, is capped and polyadenylated, and is further processed by the microprocessor complex consisting of Drosha (ribonuclease III enzyme) and DGCR8 (DiGeorge syndrome critical region 8) proteins to form precursor miRNA (pre-miRNA) having a 70–100 bp length with a 3′ end overhang [33]. The pre-miRNA is transported from the nucleus to the cytoplasm by the Exporting 5 protein, and in the cytoplasm, the 5′ and 3′ ends of the pre-miRNA hairpin are cleaved by Dicer, forming a mature miRNA duplex with about 22 bp length [33,34,35]. Among the two strands of the miRNA duplex, the one originating from the 5′ end of the pre-miRNA hairpin is called the 5p strand, and the strand originating from the 3′ end is called the 3p strand. In contrast to siRNA, miRNAs can be loaded onto the Ago protein on both strands derived from duplex, and the selection of the guide strand is influenced by its thermodynamic properties [36]. The 5p and 3p strands _target different mRNAs because they have sequences complementary to each other. When one strand of miRNA duplex is loaded onto the Ago protein, the miRNA duplex is unwound to single strands, and the unloaded strand is released and degraded. Depending on the type of Ago onto which the miRNA is loaded, it either destabilizes the _target mRNA or cleaves the _target mRNA [37]. Anti-cancer therapy approaches with synthetic miRNAs are called miRNA (miR) mimics because they make cancer cells have the same sequence as the inhibited or inactivated tumor suppressor miRNAs. miR mimics suppress cancer cells by mimicking the biological functions of the endogenous tumor inhibitor miRNAs, such as Let-7 [38], miR-29 [39], miR-34 [40], miR-101 [41], miR-126 [42], and miR-497 [43]. Chemically synthesized miR mimics can be treated in a single-stranded form, as needed, but RNAi efficiency is reduced compared to the double-stranded form, as in the case of siRNA [44]. On the other hand, single-stranded miR mimics have relatively high cell membrane permeability compared to the double-stranded miR mimics and may be useful in reducing off-_target effects [45].
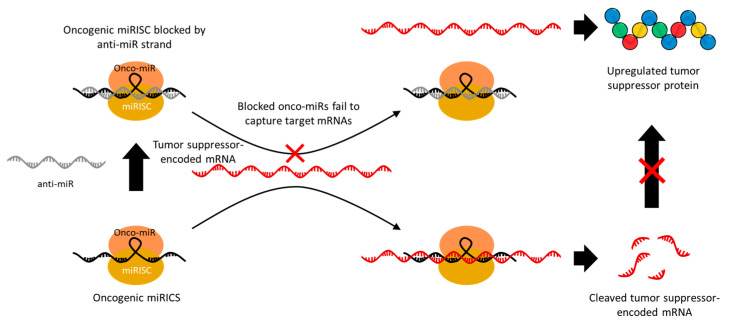
Some miRNAs act as oncogenes that _target mRNAs in tumor suppressor genes and are overexpressed in cancer. In this case, anti-miRNA (anti-miR) is used to irreversibly inhibit the gene-silencing activity of these onco-miRs [46]. Therefore, the use of anti-miRs leads to the upregulation of one or more tumor suppressor genes that were being inhibited by the _target onco-miRs, and thus they have a significant potential for anti-cancer therapy. The anti-miR sequence is designed to have a high affinity with the miRNA; thus, it competitively hybridizes to the miRNA–RISC (miRISC) along with the _target mRNA (Figure 3) [47]. The miRISCs hybridized with anti-miR can no longer cleave the _target mRNA, resulting in an upregulation of the _target gene expression. Among various oncogenic miRs, miR-21 in particular is upregulated in various types of cancer and has therefore been widely _targeted in anti-miR-based cancer suppression studies [48,49,50,51]. miR-21 mainly causes tumor development, growth, and metastasis by downregulating the genes related to apoptosis, such as phosphatase and tensin homolog (PTEN) and programmed cell death protein 4 (PDCD4), as well as tumor growth and metastasis inhibitory genes, such as reversion-inducing cysteine-rich protein with Kazal motifs (RECK) and tropomyosin 1 (TPM1) [52,53]. In addition, miR-155 [54], miR-191 [55], miR-203 [56], miR-214 [57], and miR-221 [58] are also known to downregulate major tumor suppressor genes, which makes them a promising _target for anti-miR therapeutics. Since one miRNA can effectively regulate the expression of multiple _target proteins, the delivery of miR mimics related to tumor suppression or anti-miRs that block tumor-induced miRNAs has great potential for anti-cancer therapy.
Figure 3.
Schematic image of the mechanism of anti-miRs inducing upregulation of tumor suppressor genes by blocking oncogenic miRISC. Oncogenic miRICS inhibits expression by cleaving tumor suppressor-encoded mRNAs. Anti-miRs hybridize to oncogenic miRICS and block the gene silencing activity. As a result, tumor suppressor genes are upregulated.
2.2. Antisense Oligonucleotide (ASO)
ASO is a short, single-stranded deoxyribonucleotide with 12~24 nt in length that hybridizes by Watson–Crick base pairing with specific _target mRNAs with complementary sequences. Upon being introduced into the cytoplasm and nucleus, ASO inhibits the translation of _target mRNA, mainly through RNase H-mediated cleavage [59,60]. ASO is introduced as a single strand and hybridized to the _target RNA, resulting in the formation of an RNA–DNA heterodimer, which is cleaved by RNase H (Figure 2B). RNase H is an endonuclease that is distributed in both the nucleus and cytoplasm and specifically recognizes RNA–DNA heteroduplexes and cleaves the RNA strand selectively. ASO, with a chemically modified sugar backbone, such as 2′-O-methyl (2′-OMe) or 2′-O-methoxyethyl (2′-MOE), inhibits the translation of _target mRNA through steric hindrance because it interferes with the binding and cleavage of RNase H to the RNA–DNA heteroduplex [61,62,63]. These chemical modifications provide additional advantages in increasing the physiological stability of ASOs. Similar to siRNA, ASO _target genes are involved in cancer differentiation, growth, proliferation, and survival, such as Bcl-2 [64], survivin [65], EGFR [66], signal transducer and activator of transcription 3 (STAT3) [67], and PD-L1 [68]. Gene silencing via ASOs has an advantage over siRNAs in that it can _target precursor mRNA (pre-mRNA) or long non-coding RNA (lncRNA) within the nucleus [69,70]. lncRNAs are long non-coding RNAs with a length of greater than 200 nt involved in the regulation of gene expression through various biological mechanisms and are clearly distinguished from the highly conserved short non-coding RNAs, such as miRNAs and siRNAs [71,72]. Therefore, abnormally expressed lncRNAs are accompanied by DNA damage, immune evasion, and cellular metabolic disorders, which contribute to various diseases, including cancer [73,74,75]. Selective inhibition of abnormally expressed lncRNA via ASO is an effective approach to cancer treatment [76]. Meanwhile, pre-mRNA is one of the primary transcripts transcribed from a gene and is an mRNA that contains introns before the splicing process [77]. Sometimes, to increase the expression rate of proteins required for the disease treatment, ASOs that cause steric hindrance are hybridized to the splice site of pre-mRNA to regulate the splicing process or to the upstream open reading frames (uORFs) of mRNA [78,79,80]. In summary, this therapeutic approach uses ASO to treat cancer by suppressing oncogenic genes or increasing the expression of tumor suppressor genes by _targeting various _target RNAs present in the cytoplasm and nucleus.
2.3. Catalytic Nucleic Acid (CNA)
CNAs are short single-stranded RNA (ribozyme) or DNA (DNAzyme) molecules with biological catalytic functions, such as RNA cleavage, in combination with a metal cofactor (Figure 2C) [81,82]. A CNA-based gene-silencing strategy has the advantage of not interfering with the endogenous RNAi pathway even when treated at high concentrations because it does not rely on endogenous protein enzymes. In addition, CNAs lacking catalytic activity sometimes exhibit the gene-silencing activity through the antisense effect [83,84]. Before the discovery of CNAs, it was thought that all enzymatic processes within cells were protein-based. This assumption has changed since ribozyme was discovered in the early 1980s, in which the self-splicing RNA or the catalytic site of RNase P was an RNA moiety [85,86]. In contrast to ribozymes found in nature, DNAzymes, which first appeared in the 1990s, are selected using in vitro selection and chemically synthesized [87]. The in vitro selection process prepares a DNA library containing 1013–1016 random sequences and incubates them with the _target under the applicable conditions. In the next step, nonspecifically reacting sequences are removed, and sequences that react specifically are recovered and amplified. This process is repeated for 5–15 rounds to enrich the winner DNA strands and perform sequence analysis. Finally, the sensitivity and selectivity between the winner DNA strands are compared to select the desired DNAzyme. Ribozymes can be found not only in nature but also synthesized through in vitro selection [88]. In addition to CNAs that catalyze RNA cleavage reactions, there are CNAs with various other functions, such as alkylation, ligation, and oxidation, but this review will focus on the RNA-cleaving CNAs used in gene silencing [88,89].
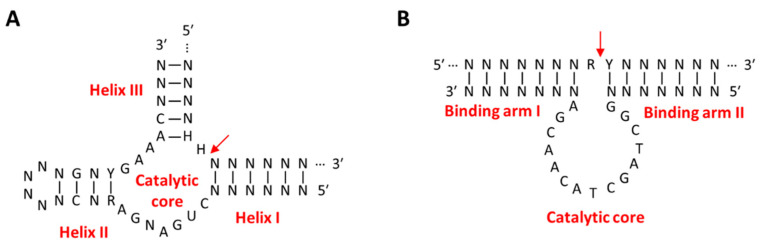
Among ribozymes, the hammerhead ribozyme (HHR) is the most extensively studied in gene therapy. The activated structure of HHR consists of a conserved catalytic core and three helixes surrounding it, out of which two helixes, helix I and III, are formed by hybridization with the _target RNA (Figure 4A) [90]. The advantages of HHR as a gene-silencing agent are that the sequences of helix I and III can be easily controlled without inhibiting the activity of the ribozyme, and the total length of the ribozyme is 35 nucleotides, making it easy to chemically synthesize [91]. HHR recognizes and binds to a _target RNA with a sequence complementary to the binding site and cleaves specific sites of _target RNA through phosphate diester isomerization. Divalent metal cations are not essential for the catalytic reaction of hammerhead ribozyme but have been reported to stabilize the activated structure [91]. Similar to other gene-silencing agents, _targets of ribozyme for anti-cancer therapy are genes that are cancer-inducing, such as mutant K-ras [92] and fibroblast growth factor-2 (FGF-2) [93], or important for cancer survival, such as γ-Glutamylcysteine synthetase (γ-GCS) [94] and multiple drug resistance 1 (MDR-1) [95]. Sometimes, ribozymes recognize cancer biomarkers, such as mutant p53 protein or human telomerase reverse transcriptase (hTERT) mRNA, and then activate cancer-killing genes, such as diphtheria toxin (DT) or herpes simplex virus thymidine kinase (HSVtk), that are delivered together to kill cancer cells directly [96,97].
Figure 4.
Schematic images of the secondary structure of (A) the hammerhead ribozyme–substrate complex and (B) the 10–23 DNAzyme–substrate complex. The red arrow indicates the cleavage site of the substrate RNA. (A) The hammerhead ribozyme consists of an intrastrand helix (helix II) and two interstrand helixes (helix I and III) generated after hybridizing with substrate RNA and a conserved unpaired catalytic core. (B) The 10–23 DNAzyme consists of two variable binding arms, I and II, on both sides of the conserved (15 nt) and unpaired catalytic core. R represents A or G; Y represents U (or T) or C; H represents A, C, or U; N represents A, U (or T), C, or G.
RNA-cleaving DNAzymes (RCDs) are suitable for use as gene-silencing agents because they are physiologically stable compared to ribozymes, can cleave _target mRNA more precisely, and are cost-effective to synthesize compared to other nucleic acid therapeutics [98]. Similar to ribozyme, RCDs have binding arms that can bind to substrate RNA on both sides of the conserved catalytic core (Figure 4B). RCDs cleave the substrate RNA bound to the binding arm through a transesterification reaction with the assistance of a divalent metal cation bound as a cofactor to the catalytic core [99]. The 10–23 RCD is suitable for intracellular gene silencing because it can be designed to cleave almost all _target mRNAs with purine–pyrimidine (R-Y) junctions utilizing Mg2+ ions as cofactors with little effect on catalytic function [100,101,102]. Anti-cancer strategies using RCDs work by _targeting genes involved in cancer differentiation, growth, and proliferation, such as Bcl-2 [103], survivin [104], early growth response protein 1 (EGR1) [105], and urokinase plasminogen activator surface receptor (uPAR) [106], or genes involved in cancer survival, such as PD-L1 [107], c-jun [108], and insulin-like growth factor II promoter 3 (IGF-IIP3) [109]. RCDs have a unique advantage over other gene-silencing agents in that their gene-silencing efficacy can be improved by adjusting the length of the binding arm, chemical modification, or using a combination of multiple RCDs [110,111,112,113]. In addition, RCDs are able to activate the catalytic core by oncogenic mRNA to simultaneously achieve down-regulation of the _target mRNA, additional anti-cancer strategies, and diagnosis of cancer [114,115]. However, since CNAs are less efficient in gene silencing compared to other endogenous gene-silencing pathways, they have recently been mainly used as a trigger for drug release or as a signal generation tool for disease diagnosis.
To summarize, anti-cancer therapies through gene silencing introduced above commonly go through the following steps: (1) identifying genes involved in cancer development, (2) designing and synthesizing NATs that specifically inhibit cancer-related genes, and (3) safely delivering the synthesized NATs to the cytoplasm of _target cells. _target genes include genes related to cancer growth, differentiation and metastasis, angiogenesis, immune avoidance, and drug resistance.
3. Aptamer
Aptamers are single-stranded RNA or DNA oligonucleotide molecules, 20 to 100 nucleotides long, that fold into specific three-dimensional structures to specifically recognize and bind specific _targets. In addition to their specific three-dimensional folding structure complementarity, aptamers also provide high selectivity and binding affinity with specific _targets through noncovalent interactions, such as hydrogen bonding, van der Waals forces, electrostatic interactions, hydrophobic effects, and π-π stacking [116,117]. Aptamers, also known as chemical antibodies, are similar to conventional antibodies consisting of proteins in that they not only bind to their _targets but can also modulate the biological activities of the _targets [118]. This suggests that aptamers can act as bioregulators and be utilized as therapeutic agents for anti-cancer therapy. In addition, aptamers have advantages over conventional antibodies, such as high thermal and physiological stability, low immunogenicity, small size, low production cost, and easy functionalization [119]. In particular, aptamer can be integrated with therapeutic factors, such as small-molecule chemo drugs, peptide molecules, and other NATs, as molecular recognition factors and can be utilized for _targeted cancer treatment [120].
The first RNA aptamer was reported by Ellington et al. in 1990 and was successfully screened through the SELEX process [121]. In 1992, the first DNA aptamer was reported by Bock et al., and since then, many aptamers have been screened through SELEX [122,123]. SELEX to obtain aptamers is similar to the SELEX process of CNA. Combinatorial DNA/RNA libraries containing 1015–1016 different random sequence regions (20–60 nt) are incubated with the _target followed by isolating and amplifying sequences that bind to the _target, and repeating this process in 8–12 rounds to ultimately obtain sequences with high affinity for the _target. As various SELEX technologies have been developed, a variety of aptamers have been screened for various _targets, such as small molecules, peptides, and proteins [116]. In particular, significant progress has been made in the field of nucleic acid-based anti-cancer therapeutics by selecting aptamers that _target cancer cells via cell-based SELEX [124]. In the case of aptamers, there was no difference in effectiveness between RNA aptamers and DNA aptamers, and DNA aptamers are known to be advantageous in terms of synthesis process and physiological stability [125].
Aptamer-based anti-cancer therapeutic strategies can be broadly divided into three categories: (1) interfering with the biological function of _target proteins, (2) enhancing cancer immunotherapy, and (3) integrated with therapeutic agents for _targeted therapy.
3.1. Aptamer-Based Protein Inhibition
The anti-cancer strategy of interfering with the biological function of a _target protein involves inhibiting the growth, metastasis, and invasion of cancer by binding to growth factor receptors or chemokine receptors to block cell signaling [126]. Platelet-derived growth factor (PDGF) is one of the growth factors that regulates cell growth and division. Since it especially regulates angiogenesis, which is essential for cancer growth, the overexpression of PDGF is found in various cancers. Therefore, PDGF was considered a promising _target for cancer treatment, and in fact, blockade of the interaction between PDGF and PDGF receptor through aptamer resulted in the inhibition of colon cancer proliferation [127,128]. CXC motif chemokine 12 (CXCL12) is a chemokine protein, also known as stromal cell-derived factor 1 (SDF-1), that provides cancer drug resistance and immune checkpoint inhibitor resistance. NOX-A12 is an aptamer for CXCL12, which binds to CXCL12 and interferes with its interaction with CXCR4 and CXCR7 receptors [129]. When NOX-A12 was used in combination with radiotherapy, immune checkpoint inhibitors, or cytotoxic drugs, effective inhibition of cancer growth was observed [129,130,131]. The overexpression of the human epidermal growth factor receptor (HER2) is also associated with cancer growth and survival, and tumor suppression can be achieved through protein interference with aptamers _targeting HER2 [132,133]. The protein inhibition efficiency of these aptamers eventually depends on the affinity between the aptamers and _target proteins; hence, multiplexing of aptamers has been shown to be more effective in inhibiting surface proteins, such as HER2, PDGF, and epithelial cell adhesion molecules (EpCAM) (Figure 5A) [133,134].
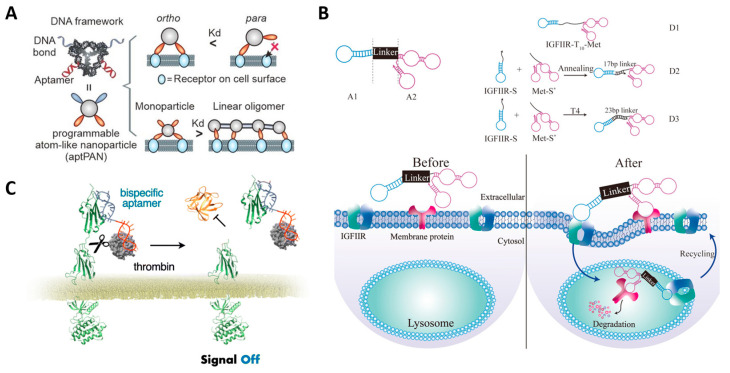
In addition to strategies in which aptamers bind to _target proteins and interfere with their interactions, bispecific aptamers can be utilized to control cell signaling by regulating the spatial arrangement of cell surface receptors. This strategy allows for more precise _targeting because it _targets multiple types of cancer-related proteins simultaneously, thereby minimizing any off-_target effects [135]. The mesenchymal epithelial transition (Met) receptor is considered one of the _targets for preventing cancer metastasis because it is known to be involved in cell growth and specifically in the metastasis of cancer cells. Studies have reported that pairing the Met receptor aptamer with a cancer cell biomarker, CD71 or Transferrin receptor (TfR), using a bispecific aptamer interferes with the interaction between the Met receptor and hepatocyte growth factor (HGF), thereby inhibiting cancer cell metastasis [136,137]. Han et al. reported a platform technique for inducing lysosomal degradation of _target proteins by utilizing bispecific aptamers that simultaneously _target Met receptors and lysosome-shuttling receptors (IGF-IIRs) (Figure 5B) [138]. Hoshiyama et al. achieved the inhibition of tumor growth signaling through aptamer-mediated cleavage using a bispecific aptamer that simultaneously _targets fibroblast growth factor receptor 1 (FGFR1) and thrombin, a protease that selectively cleaves Arg–Gly bonds (Figure 5C) [139].
Figure 5.
Anti-cancer therapeutic strategies using aptamer-based protein inhibition. (A) Schematic image of multimeric aptamer for EpCAM and PDGF protein inhibition. (B) Schematic image of bispecific aptamer for lysosomal degradation of _target membrane proteins with IGFIIR-mediated lysosomal proteolysis systems. (C) Schematic image of bispecific aptamer for _target membrane receptor inhibition by thrombin-mediated cleavage. (Adapted from Refs. [134,138,139]).
3.2. Aptamer-Based Immunotherapy
Immunotherapy is a treatment for cancer that stimulates the immune system to induce immune cells to attack cancer cells, unlike conventional cancer treatments that directly attack cancer [140]. When cancer cells appear, they are recognized by lymphocytes, including natural killer (NK) cells and T cells, and these immune cells kill cancer cells by secreting cytokines, such as Interferon-γ, and cytotoxic proteins, such as perforin, which is called tumor immune surveillance [141]. Cancer cells actively resist immune cells and ultimately escape immune surveillance through an immune editing process in which cancer cells with low immunogenicity survive and grow [142]. Immunotherapy is an anti-cancer therapy that induces cancer cell death by immune cells through neutralizing the immune suppression or immune evasion mechanisms of cancer cells or enhancing the tumor recognition ability of immune cells [143]. The major aptamer-based anti-cancer therapeutic strategies can be divided into two categories: (1) aptamers that inhibit immune checkpoints of cancer cells and (2) aptamers that improve the ability of immune cells to _target cancer cells.
The immune checkpoint is an immunosuppressive pathway that regulates the antigen recognition of immune cells to prevent the indiscriminate destruction of normal cells by excessive immune responses and is an important regulator of the immune system. PD-1/PD-L1 and CTLA-4/CD80(86) are major _targets of aptamers for immune checkpoint inhibition, and immune checkpoints in cancer cells are well-known pathways for cancer to evade immune surveillance [144]. Programmed cell death 1 (PD-1) is expressed on lymphocytes, including T cells and NK cells, and inactivates lymphocytes when it interacts with PD-L1 of cancer cells. Cytotoxic T lymphocyte antigen-4 (CTLA-4) is also a T cell surface receptor that suppresses immune responses when bound to CD80 or CD86. Therefore, blocking of the PD-1/PD-L1 or CTLA-4/CD80(86) interaction via aptamer upregulates inflammatory cytokine secretion and inhibits tumor growth (Figure 6A) [145,146,147].
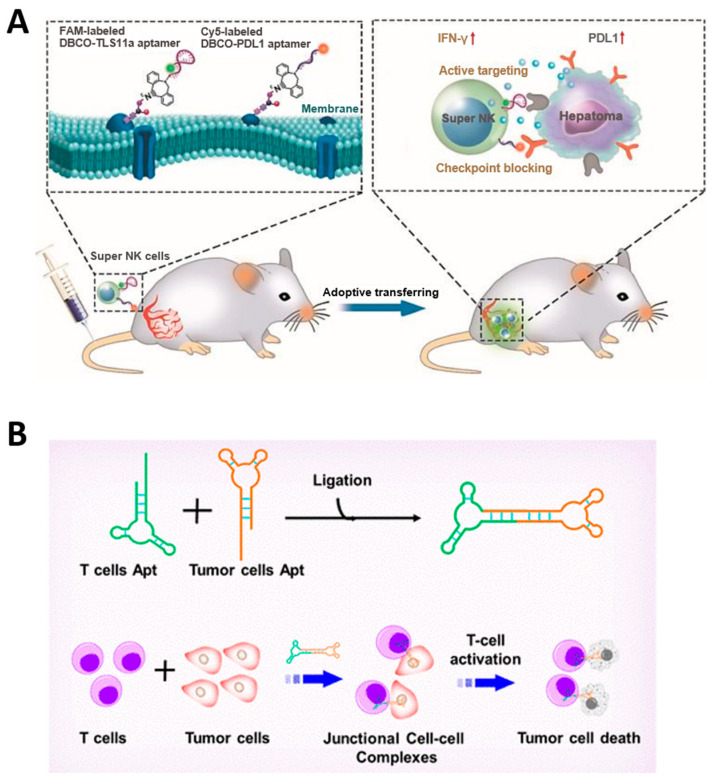
Immunomodulating bispecific aptamers that simultaneously _target cancer cells and immune cells have the effect of recruiting immune cells around the tumor cells and accumulating them at the tumor site (Figure 6B). Fcγ receptor III (CD16) of NK cells and L-selectin (CD62L) of T cells can be _targets of aptamers for recruiting immune cells, and membrane receptors overexpressed in cancer cells, such as PTK7 [148] and c-Met [149] or PD-L1 [150], an immune checkpoint, can be _targets of aptamers for binding to cancer cells. _targeting PD-L1 can inhibit the immune avoidance pathway of cancer cells, further increasing the immunotherapy effect of bispecific aptamers. These immunomodulating bispecific aptamers can be categorized with [m + n] nomenclature, where [m] represents the valency of the tumor _target aptamer and [n] represents the valency of the immune cell _target aptamer, and higher order valencies indicate not only higher acquired affinity up to a specific upper limit but additionally acquired resistance to nucleases through steric hindrance [151].
Figure 6.
Anti-cancer therapeutic strategies using aptamer-based immunotherapy. (A) Schematic image of aptamer-based immunotherapy that induces apoptosis of cancer cells by blocking immune checkpoints. (B) Schematic image of bispecific aptamer that mediates cancer cell recognition of T cells. (Adapted from Refs. [146,149]).
3.3. Aptamer-Based _targeted Therapy
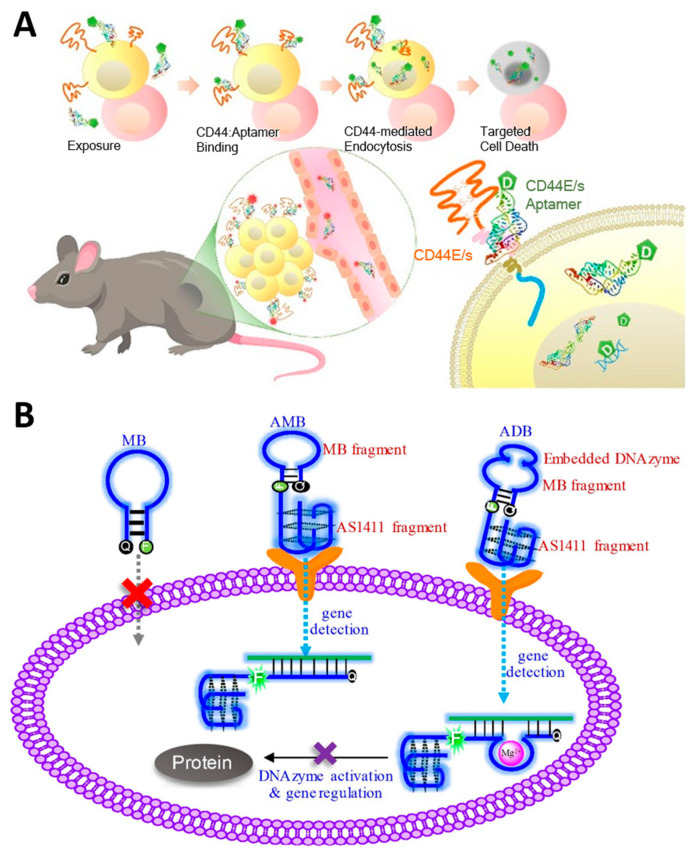
The molecular recognition properties of aptamers to _target specific _target proteins can be combined with other therapeutic elements and used in cancer-_targeting drug delivery systems. Aptamers, which can be chemically synthesized, are attractive candidates for tumor-_targeting therapy as an alternative to conventional antibodies because they can be produced inexpensively and with high reproducibility, and they facilitate the functionalization of therapeutic elements at desired locations [152]. Conventional chemotherapy and radiotherapy, which have problems, such as short half-life, high side effects, immune responses, and insufficient therapeutic effects, can have a greatly improved therapeutic effect through combination with aptamer [125,153]. Lo et al. reported an aptamer-based therapeutic that effectively inhibits hepatocellular carcinoma by conjugating the anti-cancer drug 5-fluorouracil (5-FU) to an aptamer that dually _targets CD44E/s, a surface glycoprotein overexpressed in cancer cells (Figure 7A) [154]. Jeong et al. developed an aptamer-based therapeutic that selectively inhibits breast cancer by conjugating maytansinoid (DM1), a selective microtubule inhibitor, to an aptamer _targeting HER2 [155]. Doxorubicin (Dox) is one of the most widely used anti-cancer drugs for various solid and metastatic tumors, especially due to its intercalation properties in the nucleic acid duplex; it damages the genes in the nucleus to induce apoptosis of cancer cells, and when used in combination with NATs, it has the advantage that no additional bonding process is required [156]. Yao et al. reported an aptamer-based therapeutic that can effectively _target colorectal cancer by self-assembling four AS1411 aptamers that _target nucleolin overexpressed in cancer and intercalating Dox [157]. Tan et al. reported an aptamer-based breast cancer treatment that combines a chemosensitizer and an anti-cancer agent by co-delivering a peptide that specifically inhibits heat shock protein 70 (HSP70), a cytoprotective protein overexpressed in cancer, an aptamer that _targets the oncoprotein mucin-1 (MUC-1), and Dox [158]. Omer et al. used trimers of A9g aptamer _targeting PSMA to deliver Dox, thereby increasing the affinity for _target cancer cells and resulting in higher efficacy in anti-cancer effects than when using A9g aptamer monomers [159]. Aptamer is also conjugated with radiosensitizers, such as 1,10-Phenanthroline and gold nanoclusters, increasing the radiation sensitivity of tumors and enabling radiation therapy with fewer side effects [160,161].
In addition to these chemo drugs or metal nanoclusters, aptamers can be used with other NATs, such as siRNAs, ASOs, or DNA genes. In particular, aptamers have the advantage that no additional process is required for conjugation with other NATs because they consist of nucleic acid. Dassie et al. reported significant inhibition of PSMA-positive tumors when treated with a conjugating RNA aptamer _targeting prostate-specific membrane antigens (PSMA), specifically expressed in prostate cancer cells with siRNA _targeting PLK1 mRNA [162]. Wang et al. conjugated the AS1411 aptamer that _targets nucleolin and DNAzyme that recognizes and cleaves survivin mRNA and introduced a pair of fluorophore and quencher at both ends of the DNAzyme to enable simultaneous silencing and detection of tumor genes (Figure 7B) [163]. Strategies to attach two or more NATs to aptamers have also been attempted. Liu et al. reported a chimeric structure that combined an RNA aptamer _targeting PSMA with two siRNAs _targeting survivin and EGFR, demonstrating the therapeutic effect of the combined siRNA [164]. Hong et al. reported a chimeric construct in which two ASOs were introduced into the AS1411 aptamer by conjugating an ASO _targeting the oncogenic gene galectin-1 and a G-rich sequence that can form AS1411 through G-quadruplex (G4) interaction [165]. In addition, anti-miR, which blocks onco-miR, is conjugated to an aptamer _targeting cancer-related membrane proteins, such as EGFR and CD133 and is used as a _target anti-cancer treatment [48,49].
Figure 7.
Anti-cancer therapeutic strategies using aptamer-based _targeted therapy. (A) Schematic image of 5-Fu conjugated CD44E/s bispecific aptamer-based therapeutic for _targeted delivery of anti-cancer drugs. (B) Schematic image of DNAzyme-embedded AS1411 aptamer that simultaneously detects and suppresses tumor genes. (Adapted from Refs. [154,163]).
4. CRISPR Cas9 Guide RNA (gRNA)
CRISPR Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats associated protein 9) is an immune defense system naturally present in prokaryotes, such as bacteria and archaea [166,167]. The gRNA, also called sgRNA (single guide RNA), is a short RNA strand containing a 20 nt sequence complementary to the _target DNA that complexes with the Cas protein and serves as a guide for gene editing. Unlike the first- and second-generation genome-editing tools, ZFN (Zinc Finger Nuclease) and TALEN (Transcription Activator-Like Effector Nuclease), which require protein engineering depending on the _target, the third-generation tool, the CRISPR Cas system, is based on RNA-guided nucleases; hence, Cas proteins can be generally used regardless of the _target, revolutionizing genome editing [168,169]. Among the CRISPR Cas systems, class II CRISPR Cas9 is most widely used in the field of gene editing because of its simplicity, efficiency, and ease of use [170].
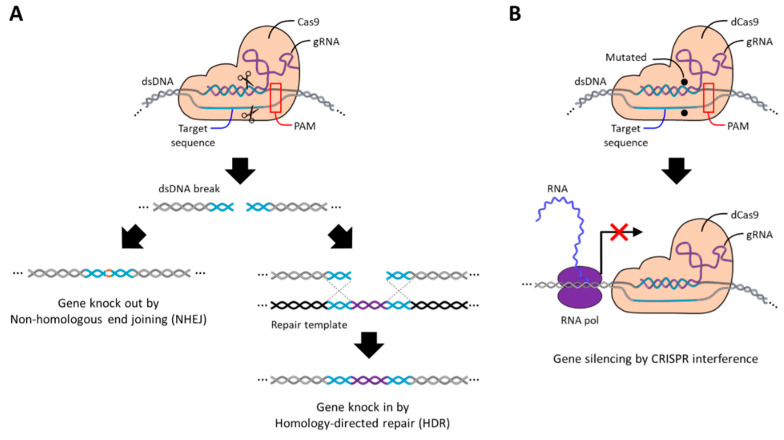
Cas9 recognizes and cleaves _target DNA, requiring a short DNA sequence 3–5 nt in length called the protospacer-adjacent motif (PAM), and compatible PAM sequences differ depending on the origin of Cas9. When gRNA hybridizes to the _target DNA, the double-stranded DNA is cleaved to have blunt ends by endonuclease domains called RuvC and HNH contained in the Cas9 protein [171]. After that, the cleaved DNA site, also called double-stranded break (DSB), is repaired by a defense mechanism of cells against DNA damage called non-homologous end joining (NHEJ) or homology-directed repair (HDR) [169,172]. In the absence of additional repair templates, cells generally repair DSB through the NHEJ pathway, during which nucleotides are randomly added or removed, resulting in insertion or deletion (indel) errors that knock out _target genes (Figure 8A). On the contrary, in the presence of a repair template, DSB is repaired by inserting a new gene through the HDR pathway, which is called gene knock-in. In addition to gene editing, gene silencing can be induced by suppressing the transcription of _target genes through an inactive Cas9 (dCas9) lacking endonuclease activity due to the D10A and H840A mutations [173,174]. dCas9 can still hybridize with the _target DNA via gRNA, but it is not able to cleave the DNA strand so it remains bound to the _target gene. As a result, the transcription process of RNA polymerase for the _target gene is sterically blocked by dCas9, thereby silencing the _target gene (Figure 8B).
Figure 8.
Schematic images of the CRISPR Cas9 and CRISPR dCas9 systems. (A) Schematic image of CRISPR Cas9 genome editing mechanism. The endonuclease domains of Cas9 (RuvC and HNH) cleave the _target sequence after hybridization, and the cut DNA is repaired by NHEJ or HDR, which results in gene disruption or replacement. (B) Schematic image of CRISPR dCas9 gene-silencing mechanism. Mutations in the nuclease domain of dCas9 (D10A and H840A) render it unable to cleave the _target sequence after hybridization and interfere with the RNA transcription process through steric hindrance.
Through CRISPR Cas9, the anti-tumor effect can be greatly improved through knockout of cancer-causing genes, such as DNA methyltransferase 1 (DNMT1) gene [175], survivine gene [176], and EGFR gene [177]. Additionally, oncogenic miRNAs that inhibit tumor suppressor genes may also be promising _targets of CRISPR Cas9 for anti-cancer therapy [178,179,180]. CRISPR Cas9 can also be utilized to select promising _target miRNAs with anti-cancer effects [181]. Jiang et al. demonstrated that the knockout of miR-205, miR-221, miR-222, miR-30c, miR-224, miRNA-455-3p, miR-23b, and miR-505 through the CRISPR Cas9 system was effective in inhibiting the growth of prostate cancer (PCa). Genes involved in drug resistance are also promising _targets for CRISPR Cas9, which exhibits effective anti-cancer activity [182]. Gene editing or gene knockout utilizing the CRISPR Cas9 system is a unique cancer treatment strategy that is difficult to achieve with other NATs. However, unlike other NATs that utilize the function of endogenous protein or the NAT itself, there are obstacles as the Cas9 protein must be delivered together with the gRNA, and it can cause fatal side effects due to irreversible DNA sequence alteration. The safety and ethical issues of CRISPR Cas9-based gene editing technology are a topic of concern for many experts, and discussions and debates surrounding them are still ongoing [183,184,185]. Despite many improvements, the gRNA of CRISPR Cas9 can form unusual hybridization with non-complementary sequences, which may lead to irreversible and lethal off-_target effects. In addition, rare deletions of multiple genes have been reported during repair via the NHEJ pathway, which may lead to the development of cancer. In addition, there are ethical concerns that gene editing techniques could be applied to human germ cells to design a baby’s genetic traits.
5. Gene Expression
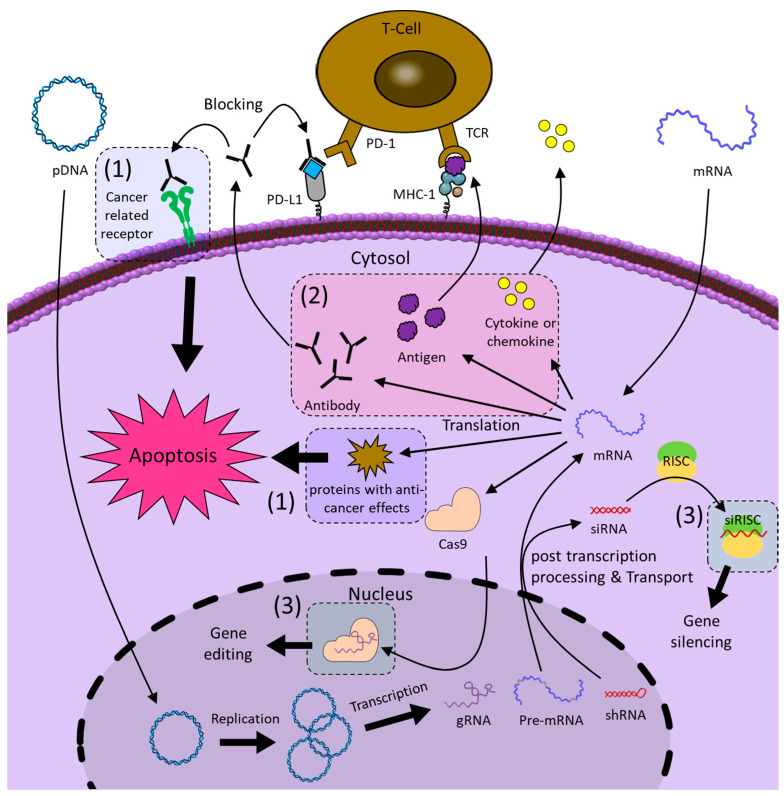
Gene expression is the process of creating a protein with biological functions through the flow of information from DNA to RNA and from RNA to protein. Both pDNA and mRNA are genetic vehicles that are delivered into cells to express the encoded genes. Anti-cancer therapy through gene expression of pDNA can be broadly divided into three types: (1) the expression of proteins with anti-cancer effects, (2) DNA vaccines that induce immunotherapy by expressing antigens or checkpoint inhibitors, and (3) the expression of RNA-based NATs (Figure 9). pDNA therapeutics must contain desired genetic information, and they have to reach the nucleus where RNAs can be expressed [186,187]. This transfection step is a difficult process that may reduce the therapeutic efficacy of pDNA therapeutics. On the other hand, mRNA therapeutics do not need to reach the nucleus because they can express proteins in the cytoplasm. In the case of mRNA anti-cancer therapy, it is divided into (1) tumor suppressor protein expression and (2) mRNA cancer vaccine (Figure 9). However, mRNA therapeutics have lower physiological stability than pDNA therapeutics and have a shorter duration of therapeutic effect than pDNA, which continuously produces RNA after being transfected [188].
Figure 9.
Schematic image of the anti-cancer mechanisms of pDNA and mRNA therapeutics. pDNA is delivered to the nucleus and replicated or transcribed by enzymes to synthesize RNA with therapeutic effects, such as gRNA, pre-mRNA, or shRNA, depending on the genes inserted. Pre-mRNA and shRNA are post-transcriptionally processed by enzymes into mRNA and siRNA, respectively, and transported out of the nucleus. mRNA is transported into the cytoplasm and translated by ribosomes to synthesize the encoded proteins, such as antigens, antibodies, cytokines, tumor suppressor proteins, and Cas9 proteins. The numbers represent (1) expression of a protein with anti-cancer effects, (2) DNA vaccine that induces immunotherapy by expressing an antigen or checkpoint inhibitor, and (3) expression of RNA-based NATs.
5.1. Plasmid DNA (pDNA)
Plasmid DNA (pDNA) is a small circular DNA molecule found in bacteria that replicates independently of the genome and expresses genes. For therapeutic utilization of pDNA, the optimization of the plasmid backbone, including the insertion site of the desired genetic information and the arrangement of key elements, such as promoters and internal ribosome entry site (IRES), is important, which affects the immunogenicity and therapeutic effects of pDNA [187]. Transfecting the protein-encoding plasmid DNA, which has a direct anti-cancer effect, into _target cancer cells can effectively suppress cancer cells (see (1) in Figure 9). Candidate proteins with such anti-cancer effects include proapoptotic proteins [189]; tumor suppressor proteins, such as p59 [190], PTEN [191], and PDCD4 [192]; cytotoxic proteins, such as saporin (SAP) [193]; proteins that enhance immunogenicity, such as RNA replicase [194]; bacterial toxins, such as diphtheria toxin [195]; and inhibitors of cancer-related receptors, such as HER2 antibody [196] and PSMA antibody [197]. Another anti-cancer strategy utilizing pDNA can induce the destruction of the cancer with the immune system by generating specific proteins called DNA cancer vaccines (see (2) in Figure 9). DNA vaccines are considered safer and more effective treatment strategies than conventional vaccines because they directly transmit the genetic information of antigens [198]. DNA vaccines use pDNA that encodes cytokines, such as interferons (INF-β, INF-γ) [199] and tumor necrosis factor-α (TNF-α) [200]; antibodies to block immune checkpoints, such as PD-L1 [201] and PD-1 [202]; and antigens, such as ovalbumin (OVA) [199], TERT [203], and glycoprotein-100 (gp-100) [204]. Next, pDNA therapeutics can be designed to express RNA-based NATs through their property to transcribe RNA after transfection (see (3) in Figure 9). short hairpin RNA (shRNA), which has an RNAi mechanism similar to siRNA, is one of the RNA-based NATs transcribed in the cell nucleus from pDNA and consists of 19–22 bp double-stranded RNA linked by short hairpins [205]. The shRNA is treated with siRNA by dicer in the cytoplasm and subsequently complexed with RISC to exhibit gene-silencing activity. pDNA encoding shRNA that silences genes involved in cancer survival and growth, such as AKT Serine/Threonine Kinase 1 (Akt1) [192], Vascular Endothelial Growth Factor (VEGF) [206], and EGFR [207], exhibits anti-cancer effects. Additionally, pDNA encoding both Cas9 protein and gRNA is a very simple and convenient strategy that avoids repetitive transfection of each component of the Cas9 system [170]. Zhao et al. achieved permanent immune checkpoint inhibition by encoding gRNA _targeting the PD-L1 gene and Cas9 proteins in pDNA and observed the resultant activation of T-cell-mediated anti-tumor responses [208]. However, these strategies raise concerns that the long-lasting activity of Cas9 may increase off-_target effects compared to strategies that directly deliver Cas9-gRNA complexes [209].
5.2. Messenger RNA (mRNA)
Messenger RNA (mRNA) is a type of RNA that carries genetic information that can direct the synthesis of proteins, and therefore it is the _target of many NATs to silence genes. However, mRNA encoding for therapeutic proteins can be used as therapeutics. Unlike pDNA therapeutics, mRNA therapeutics do not need to enter the nucleus to function, making transfection relatively easy and safe [210]. In addition, mRNA therapeutics have great potential for personalized medicine because they can be produced at lower production costs in a shorter period of time than pDNA therapeutics [211]. mRNA therapeutics are considered an alternative to pDNA therapeutics, which have low transfection efficiency and can cause permanent side effects [212]. mRNA therapeutics production involves multiple unit operations, including pDNA preparation, pDNA linearization, in vitro mRNA transcription (IVT) reaction, pDNA digestion, and mRNA purification [213]. The completed mRNA therapeutic contains a 5′ cap, a 3′ poly adenine (A) tail at both ends to enhance mRNA physiological stability and protein translation efficiency, and a 5′ untranslated region (UTR), a gene of interest (GOI), and a 3′ UTR are located between them. Similar to pDNA, protein-encoded mRNAs with anti-cancer effects induce cancer cell apoptosis (see (1) in Figure 9). mRNA encoding tumor inhibitors, such as P53 [214], PTEN [215], tuberous sclerosis complex 2 (TSC2) [216], mothers against decapentaplegic homolog 4 (SMAD4) [217]; proteins that induce cell membrane disruption, such as mixed lineage kinase domain-like protein (MLKL) [218]; or cancer-related receptor inhibitors, such as HER2 antibody [219], exhibit direct anti-cancer effects. The mRNA cancer vaccine, which induces anti-tumor activity by the immune system, is currently one of the most actively studied treatments [220]. As cancer vaccines, mRNA encoding cytokines, such as Interleukin-12 (IL-12), IL-27 [221], IL-23, IL-36γ [222], and INF-α [223]; antigens, such as OX40L [219], OVA [224], cytokeratin 19 (CK19) [225], and MUC1 [226]; or antibodies that block immune checkpoints, such as anti-PD-1 and anti-PD-L1 [227], can be used (see (2) in Figure 9). In addition, mRNA encoded with the Cas9 protein is co-delivered with gRNA and used for gene editing. Wang et al. reported that the Cas9 protein-encoded mRNA is co-delivered with gRNAs that _target legumain (LGMN) genes, which are known to cause various diseases, including cancer, effectively suppressing the metastatic properties of breast cancer [228].
6. Delivery Strategies
Although nucleic acid-based therapies have the potential to treat a wide range of diseases, including those that are difficult to treat with conventional anti-cancer therapies, their use is limited by inherent problems, such as low physiological stability and cell membrane permeability [10,11]. Moreover, exogenous nucleic acids can induce innate immune responses and cause associated off-_target effects, making in vivo applications difficult [9,229]. Therefore, for effective and safe anti-cancer therapy employing NATs, an appropriate delivery system that isolates the NATs from the external environment and increases cell internalization efficiency is essential. In response to this requirement, various virus-based delivery systems and non-viral-based delivery systems, such as DNs, INPs, and LNPs, have been developed for NAT delivery. The development of a delivery system is the most important element in anti-cancer therapy employing NATs and has been extensively studied worldwide.
Among them, efforts to use viral vectors for NAT delivery have been around for 20 years [230]. Viral vectors are drug carriers that deliver NATs using a mechanism for transfecting genes into virus-infected cells. For this purpose, NATs are loaded into viruses that have reduced pathogenicity and immunogenicity. Despite recent advances in non-viral-based vectors, viral vectors still remain the most widely used delivery route for NATs, and most clinically approved NATs have used viral-based vectors [231]. Viral vectors have advantages, such as versatility for various _target cells, high delivery efficiency, and large loading capacity. Viral vectors protect internal NATs with a protein shell called capsids, and some enveloped viral vectors have lipid bilayers surrounding the capsids. Viral vectors continue to improve and advance despite many potential threats and have achieved great results, with many viral vector-based therapeutics passing clinical trials around adenovirus vectors (AVs) [232]. Since the AV has a genome consisting of a long double-stranded DNA of up to 36 kb, it can insert large therapeutic genes and deliver viral DNA with high efficiency. However, since the delivered viral DNA is not integrated into the genes of the infected cell, viral gene expression is transient, and there is a risk that it may be delivered to normal cells and cause off-_target effects. Furthermore, there is a disadvantage in that it exhibits strong immunogenicity [233]. In contrast, adeno-associated virus vectors (AVVs) are considered ideal vectors because they integrate into the genes of the _target cells, allowing long-term expression of therapeutic genes, and have lower pathogenicity and immunogenicity than other viral vectors, including AVs. However, they have a disadvantage, as the size of the gene that can be inserted is less than 5 kb [233]. Despite continued improvements, viral vectors remain to possess relatively high immunogenicity and limited viral lifespan as they originate from viruses and are still a potential threat to the viral vector-based NAT delivery strategies [232]. These limitations make repeated administration of viral vectors difficult and lead to a decrease in the therapeutic efficacy.
Next, since DNs consist of nucleic acids, NATs can be loaded through hybridization. DNs can be considered carriers to increase the stability and intracellular transfer efficiency of NATs [234]. DNs have unique advantages as they are easy to functionalize, can precisely control the shape and size of the structure on a nanometer scale, and can carry out reversible structural transitions in response to external environments and materials, enabling controlled release. In addition, these nanostructures are composed of DNA and therefore have excellent biocompatibility and biodegradability. In particular, tetrahedral DNA nanostructures (TDNs) are widely used as drug carriers due to their strong resistance to nuclease and receptor-mediated high cell membrane permeability, thanks to their simple structure of four oligonucleotides [235]. However, since TDN is a small structure with a diameter of less than 20 nm, it has a small loading capacity and is difficult to use for the delivery of large-sized NATs, such as mRNA or pDNA. On the other hand, DNA hydrogel (Dgel) is a DNA nanostructure with a 3D network structure, and it is mostly composed of water, so it has excellent biocompatibility, high programmability, and large loading capacity, and thus has received much attention as a drug carrier [236]. Dgel has a diameter of approximately 100–200 nm and has a large loading capacity, so it has been proven that it can be used as a carrier for delivering mRNA or pDNA into cells [237,238]. Interestingly, it has been reported that compact DNA constructs, unlike loose DNA strands, have high cell membrane permeability [239,240]. In addition, DNs can easily introduce aptamers to a desired location by including aptamer sequences in the DNA strands forming the structure, and due to the flexible structural characteristics of DNA structure, additional affinity for _target proteins randomly distributed on the membrane of cancer cells can be imparted through the introduction of multivalent aptamers [134,159]. DNA strands with specific sequences, such as i-motifs and aptamers, have the property of dynamically switching their structures depending on the external environment or _target molecules, and this can be utilized to design DNs with controllable drug release properties [241]. It has even been reported that the i-motif sequence induces a proton sponge effect, helping DNs escape from endosomes [242]. But despite these various advantages, DNs still have a limited number of in vivo and clinical applications, and the need for further elucidation of their in vivo circulation, biodistribution, cellular endocytosis mechanisms, and endosomal escape pathways remains a challenge. Although DNs are highly biocompatible, sufficient investigation is essential to confirm whether they stimulate the immune system because living organisms react sensitively to exogeneous nucleic acid materials, and DNs are relatively resistant but are degraded by nucleases.
Next, INPs are also one of the many studied and used carriers that overcome several barriers and deliver NATs into cells [243]. INPs, such as gold nanoparticles (AuNPs), can protect surface-loaded nucleic acids from nucleases through steric hindrance and improve their intra-serum stability [244]. Additionally, it increases the cellular uptake efficiency [239]. AuNPs, in particular, are in the spotlight as nucleic acid carriers because they have advantages, such as ease of size control, surface modification, and relatively low cytotoxicity [245]. Thiol-functionalized nucleic acids are commonly used to conjugate nucleic acids to the surface of AuNPs [246]. Additionally, AuNPs can provide additional properties, such as quenching of fluorescent signals, photothermal therapy, and Raman scattering through their unique optical and electronic properties by surface plasmon resonance [247]. The SPR of AuNPs can be controlled depending on the size, shape, and assembly of AuNPs, and the SPR in the near-infrared region controlled through these ways has high biological applicability with high bio permeability. However, potential problems remain, such as the limited types of functional groups used in the conjugation of AuNPs to DNA and size-dependent cytotoxicity [245,248]. In addition, AuNPs are limited in the delivery of large-sized nucleic acid molecules, such as mRNAs or pDNAs, due to the way they load cargo onto their surfaces. Meanwhile, mesoporous silica nanoparticles (MSNPs) are also used as carriers of NATs due to their excellent properties, such as a well-defined pore size, high chemical stability and biocompatibility, and high loading capacity and surface area, as well as the ability to control the release rate through pore size control and surface modification [249,250]. Moreover, since MSNPs can adjust the size of the pores according to the size of the loaded NATs, loading large-sized NATs, such as mRNA, is also possible. Dong et al. achieved intracellular mRNA delivery by premixing cationic polymer polyethylenimine (PEI) with mRNA and then loading it onto MSNPs with a size of about 300 nm and pores of about 40 nm [251]. PEI can be used to electrostatically bind NATs to INPs, including AuNPs and MSNPs, providing the additional ability to induce endosomal escape via the proton sponge effect, but has the disadvantage of exhibiting strong cytotoxicity [252]. INPs show relatively high colloidal stability during in vivo circulation, but they are effectively removed by the liver and kidneys, and localization to the lesion remains difficult.
Next, LNPs are excellent non-viral NATs delivery vehicles with excellent NATs loading efficiency, relatively high biocompatibility, high physiological stability, high cell internalization efficiency and endosomal escape efficiency, and ease of mass production [253]. Currently, LNPs are the most studied and most advanced carriers of NATs and are helping produce a number of brilliant achievements, including combination with mRNA therapeutics to prevent the spread of COVID-19. In addition, _targeted therapy with LNPs can be achieved by surface functionalization with various _targeting moieties, such as antibody aptamer peptides [253]. However, the LNP is not flawless, and there is still room for development. LNPs are generally composed of helper lipids, such as cationic or ionic lipids or lipid substances, polyethylene glycol (PEG)-lipids, and cholesterol, which change immunogenicity, cytotoxicity, physiological stability, and delivery efficiency by controlling these components [254,255]. Through additional understanding of these components, the performance of LNP as a carrier can be further optimized. LNPs are spherical particles with a diameter of typically 60–150 nm, and their internal core is formed by electrostatic interactions between a negatively charged NAT and a positively charged ionic lipid at low pH; they are formed through a microfluidic system for rapid mixing. Ionic lipids are the most important components of LNPs, which have the advantage of reducing cytotoxicity and allowing long-term biocirculation by maintaining a neutral charge at physiological pH [256]. Controlling the unsaturation or length of the lipid domain of ionic lipids, or the pka value of the head group, or the introduction of biodegradable properties have been extensively studied, and accordingly, they significantly affect the properties of LNPs, such as intracellular delivery efficiency and biocompatibility. In addition, cholesterol, a helper lipid, increases the rigidity of LNP, helping with structural stability, and phospholipid, another helper lipid, helps maintain the lipid bilayer structure of LNP, helping with endosomal escape. PEG-lipid, located on the surface of LNP, prevents aggregation between particles and prevents other biomaterials from sticking, thereby playing a role in increasing colloidal stability. Additionally, adjusting or changing their ratio also greatly affects the characteristics of LNP [257]. Additionally, _targeting moieties, such as antibodies or aptamers, can be conjugated to PEG-lipids or cholesterol and introduced to the surface of LNP, and multivalent aptamers can be employed to further increase the affinity for _target cells due to the flexible surface properties of LNP [258,259].
7. Conclusions and Prospect
Since genes are made of nucleic acids, NATs can inherently affect the information flow of genes, thereby achieving anti-cancer therapy through gene expression (pDNAs and mRNAs), gene silencing (siRNAs, miR mimics, anti-miRs, ASOs, and CNAs), and gene editing (gRNAs). pDNA and mRNA have a therapeutic effect through the expression of a gene encoded with specific base sequences, while siRNA, miR mimic, anti-miR, ASO, CNA, and gRNA have a therapeutic effect through hybridization with the _target nucleic acid with interstrand base pairing. Aptamers can form 3D structures through intrastrand base pairing of unique base sequences, and they can interact with specific _target proteins and block their functions. Therefore, all NATs can act as therapeutics by having a specific sequence without additional chemical modification. Therefore, all NATs are made up of the same components, but by controlling the sequence, they can act as therapeutics with completely different function modes. NATs have the advantage of being easy to chemically synthesize to have specific sequences and easy to conjugate with various functional moieties, from small-molecule drugs to inorganic nanoparticles. In addition, NATs can be flexibly combined with other anti-cancer therapies, such as chemotherapy, radiation therapy, immunotherapy, and even other NATs, and have shown more improved anti-cancer effects. Therefore, NATs have unrivaled potential for long-term treatment with high efficiency for a wide range of cancers.
Despite the high potential of NATs, their application is extremely limited due to the defense mechanism of the body against external genetic material. These defense mechanisms include cell membranes that are impermeable to negatively charged nucleic acids, widely distributed nucleases from blood vessels to cytoplasm, the immune system sensitive to exogenous nucleic acid molecules, and the vascular endothelial barrier that must be passed to reach the tumor site. The conditions for an ideal NATs delivery carrier include high biocompatibility, high NATs loading efficiency, protection of the nucleic acid therapeutic from the external environment during delivery, and high cell membrane penetration and endosomal escape efficiency. To meet these requirements, various drug delivery systems have been developed and verified at the cellular level or at the in vivo level. The remarkable advancement in anti-cancer treatment strategies using NATs has been possible thanks to the advancement in drug delivery systems. The field of NATs has developed rapidly since COVID-19, and mRNA cancer vaccines are receiving particular attention. Currently, viral vectors, DNA nanostructures, inorganic nanoparticles, and LNPs are widely used as carriers of nucleic acid therapy, and among them, LNPs are the most studied as carriers for mRNA vaccines. This trend is expected to continue for the time being, and many nucleic acid-based chemotherapy drugs are expected to be approved for clinical trials in the future.
Author Contributions
Conceptualization, M.L. (Minhyuk Lee) and N.P.; writing—original draft preparation, M.L. (Minhyuk Lee), M.L. (Minjae Lee), Y.S., S.K. and N.P.; writing—review and editing, M.L. (Minhyuk Lee) and N.P.; visualization, M.L. (Minhyuk Lee); supervision, N.P.; funding acquisition, N.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A2C10090071313582110600103).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gopal S., Sivaram S., Rajaraman P., Trimble E.L. Think globally about cancer. Nat. Med. 2019;25:351. [Google Scholar]
- 2.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., Bray F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L., Lou W., Wang J. Advances in nucleic acid therapeutics: Structures, delivery systems, and future perspectives in cancer treatment. Clin. Exp. Med. 2024;24:200. doi: 10.1007/s10238-024-01463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yahya E.B., Alqadhi A.M. Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sci. 2021;269:119087. doi: 10.1016/j.lfs.2021.119087. [DOI] [PubMed] [Google Scholar]
- 5.Das S.K., Menezes M.E., Bhatia S., Wang X., Emdad L., Sarkar D., Fisher P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell. Physiol. 2014;230:259–271. doi: 10.1002/jcp.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cesur-Ergün B., Demir-Dora D. Gene therapy in cancer. J. Gene Med. 2023;25:e3550. doi: 10.1002/jgm.3550. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadzada T., Reid G., McKenzie D.R. Fundamentals of siRNA and miRNA therapeutics and a review of _targeted nanoparticle delivery systems in breast cancer. Biophys. Rev. 2018;10:69–86. doi: 10.1007/s12551-017-0392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetter V.C., Wagner E. _targeting nucleic acid-based therapeutics to tumors: Challenges and strategies for polyplexes. J. Control. Release. 2022;346:110–135. doi: 10.1016/j.jconrel.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni J.A., Witzigmann D., Thomson S.B., Chen S., Leavitt B.R., Cullis P.R., Van Der Meel R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021;16:630–643. doi: 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- 10.Ingle R.G., Fang W.-J. An Overview of the Stability and Delivery Challenges of Commercial Nucleic Acid Therapeutics. Pharmaceutics. 2023;15:1158. doi: 10.3390/pharmaceutics15041158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T., Tang Y., Tao Y., Zhou H., Ding D. Nucleic acid drug and delivery techniques for disease therapy: Present situation and future prospect. Interdiscip. Med. 2024;2:e20230041. doi: 10.1002/INMD.20230041. [DOI] [Google Scholar]
- 12.Paterson B.M., Roberts B.E., Kuff E.L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc. Natl. Acad. Sci. USA. 1977;74:4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizuno T., Chou M.Y., Inouye M. A unique mechanism regulating gene expression: Translational inhibition by a complementary RNA transcript (micRNA) Proc. Natl. Acad. Sci. USA. 1984;81:1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kijima H. Therapeutic applications of ribozymes. Pharmacol. Ther. 1995;68:247–267. doi: 10.1016/0163-7258(95)02008-X. [DOI] [PubMed] [Google Scholar]
- 15.Shreya, Pandey A.K., Bhandari H.R. Gene Silencing: The Mechanism to Down Regulate the _target Gene. Int. J. Bio-Resour. Stress Manag. 2018;9:682–690. [Google Scholar]
- 16.Ratcliff F., Harrison B.D., Baulcombe D.C. A Similarity Between Viral Defense and Gene Silencing in Plants. Science. 1997;276:1558–1560. doi: 10.1126/science.276.5318.1558. [DOI] [PubMed] [Google Scholar]
- 17.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 18.Lam J.K.W., Chow M.Y.T., Zhang Y., Leung S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids. 2015;4:e252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isenmann M., Stoddart M.J., Schmelzeisen R., Gross C., Della Bella E., Rothweiler R.M. Basic Principles of RNA Interference: Nucleic Acid Types and In Vitro Intracellular Delivery Methods. Micromachines. 2023;14:1321. doi: 10.3390/mi14071321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mello C.C., Conte D. Revealing the world of RNA interference. Nature. 2004;431:338–342. doi: 10.1038/nature02872. [DOI] [PubMed] [Google Scholar]
- 21.Takeda A., Iwasaki S., Watanabe T., Utsumi M., Watanabe Y. The Mechanism Selecting the Guide Strand from Small RNA Duplexes is Different Among Argonaute Proteins. Plant Cell Physiol. 2008;49:493–500. doi: 10.1093/pcp/pcn043. [DOI] [PubMed] [Google Scholar]
- 22.Holen T., Amarzguioui M., Babaie E., Prydz H. Similar behaviour of single-strand and double-strand siRNAs suggests they act through a common RNAi pathway. Nucleic Acids Res. 2003;31:2401–2407. doi: 10.1093/nar/gkg338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi X., Gatti P., Papoian T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov. Today. 2017;22:823–833. doi: 10.1016/j.drudis.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Li K., Cai X., Fan Y., Jin M., Xie Y., Jing Z., Zang X., Han Y. Codelivery of Que and BCL-2 siRNA with Lipid–Copolymer Hybrid Nanocomplexes for Efficient Tumor Regression. ACS Biomater. Sci. Eng. 2023;9:4805–4820. doi: 10.1021/acsbiomaterials.3c00489. [DOI] [PubMed] [Google Scholar]
- 25.Vaidya S., Mohod A., Eedara A.C., Andugulapati S.B., Pabbaraja S. Synthesis and Characterization of a New Cationic Lipid: Efficient siRNA Delivery and Anticancer Activity of Survivin-siRNA Lipoplexes for the Treatment of Lung and Breast Cancers. Chem. Med. Chem. 2023;18:e202300097. doi: 10.1002/cmdc.202300097. [DOI] [PubMed] [Google Scholar]
- 26.Heris N.N., Baghani L., Khonsari F., Varshochian R., Dinarvand R., Atyabi F. Delivery of EGFR-siRNA to prostatic cancerous cells based on polydopamine coated gold nanoparticles. Int. J. Drug Deliv. Technol. 2023;87:104869. doi: 10.1016/j.jddst.2023.104869. [DOI] [Google Scholar]
- 27.Aslan M., Ozturk S., Shahbazi R., Bozdemir Ö., Zeybek N.D., Vargel İ., Eroğlu İ., Ulubayram K. Therapeutic _targeting of siRNA/anti-cancer drug delivery system for non-melanoma skin cancer. Part I: Development and gene silencing of JAK1siRNA/5-FU loaded liposome nanocomplexes. Eur. J. Pharm. Biopharm. 2024;203:114432. doi: 10.1016/j.ejpb.2024.114432. [DOI] [PubMed] [Google Scholar]
- 28.Jain D., Yadav A.K. Development of hyaluronic acid–anchored polycaprolactone nanoparticles for efficient delivery of PLK1 siRNA to breast cancer. Drug Deliv. Transl. Res. 2023;13:1730–1744. doi: 10.1007/s13346-022-01288-2. [DOI] [PubMed] [Google Scholar]
- 29.Haghiralsadat F., Amoabediny G., Naderinezhad S., Zandieh-Doulabi B., Forouzanfar T., Helder M.N. Codelivery of doxorubicin and JIP1 siRNA with novel EphA2-_targeted PEGylated cationic nanoliposomes to overcome osteosarcoma multidrug resistance. Int. J. Nanomed. 2018;13:3853–3866. doi: 10.2147/IJN.S150017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao D., Che J., Han S., Zhao H., Zhu Y., Zhu H. RNAi-mediated knockdown of the CLN3 gene inhibits proliferation and promotes apoptosis in drug-resistant ovarian cancer cells. Mol. Med. Rep. 2015;12:6635–6641. doi: 10.3892/mmr.2015.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Chen Y., Liao M., Yu S., Yuan B., Jia Z., Zhou L., Tang Y. Exosomes-delivered PD-L1 siRNA and CTLA-4 siRNA protect against growth and tumor immune escape in colorectal cancer. Genomics. 2023;115:110646. doi: 10.1016/j.ygeno.2023.110646. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denli A.M., Tops B.B.J., Plasterk R.H.A., Ketting R.F., Hannon G.J. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 34.Bohnsack M.T., Czaplinski K., Görlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida T., Asano Y., Ui-Tei K. Modulation of MicroRNA Processing by Dicer via Its Associated dsRNA Binding Proteins. Non-Coding RNA. 2021;7:57. doi: 10.3390/ncrna7030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meijer H.A., Smith E.M., Bushell M. Regulation of miRNA strand selection: Follow the leader? Biochem. Soc. Trans. 2014;42:1135–1140. doi: 10.1042/BST20140142. [DOI] [PubMed] [Google Scholar]
- 37.Hutvagner G., Simard M.J. Argonaute proteins: Key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 38.Tristán-Ramos P., Rubio-Roldan A., Peris G., Sánchez L., Amador-Cubero S., Viollet S., Cristofari G., Heras S.R. The tumor suppressor microRNA let-7 inhibits human LINE-1 retrotransposition. Nat. Commun. 2020;11:5712. doi: 10.1038/s41467-020-19430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin L., Bao Y., Tian M., Ren Q., Zhang W. miR-29 family inhibited the proliferation and migration of lung cancer cells by _targeting SREBP-1. Mol. Cell. Toxicol. 2021;18:165–175. doi: 10.1007/s13273-021-00180-3. [DOI] [Google Scholar]
- 40.Zhao Y., Ye L., Yu Y. MicroRNA-126-5p suppresses cell proliferation, invasion and migration by _targeting EGFR in liver cancer. Clin. Res. Hepatol. Gastroenterol. 2020;44:865–873. doi: 10.1016/j.clinre.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 41.Kapadia C.H., Ioele S.A., Day E.S. Layer-by-layer assembled PLGA nanoparticles carrying miR-34a cargo inhibit the proliferation and cell cycle progression of triple-negative breast cancer cells. J. Biomed. Mater. Res. Part A. 2019;108:601–613. doi: 10.1002/jbm.a.36840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y., Tan J., Ou S., Chen J., Chen L. MicroRNA-101-3p suppresses proliferation and migration in hepatocellular carcinoma by _targeting the HGF/c-Met pathway. Investig. New Drugs. 2019;38:60–69. doi: 10.1007/s10637-019-00766-8. [DOI] [PubMed] [Google Scholar]
- 43.Jeong K., Yu Y.J., You J.Y., Rhee W.J., Kim J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab A Chip. 2020;20:548–557. doi: 10.1039/C9LC00958B. [DOI] [PubMed] [Google Scholar]
- 44.Matsui M., Prakash T.P., Corey D.R. Argonaute 2-dependent Regulation of Gene Expression by Single-stranded miRNA Mimics. Mol. Ther. 2016;24:946–955. doi: 10.1038/mt.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lima J.F., Cerqueira L., Figueiredo C., Oliveira C., Azevedo N.F. Anti-miRNA oligonucleotides: A comprehensive guide for design. RNA Biol. 2018;15:338–352. doi: 10.1080/15476286.2018.1445959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Rooij E., Purcell A.L., Levin A.A. Developing MicroRNA Therapeutics. Circ. Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 47.Hogan D.J., Vincent T.M., Fish S., Marcusson E.G., Bhat B., Chau B.N., Zisoulis D.G. Anti-miRs Competitively Inhibit microRNAs in Argonaute Complexes. PLoS ONE. 2014;9:e100951. doi: 10.1371/journal.pone.0100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shu D., Li H., Shu Y., Xiong G., Carson W.E., Haque F., Xu R., Guo P. Systemic Delivery of Anti-miRNA for Suppression of Triple Negative Breast Cancer Utilizing RNA Nanotechnology. ACS Nano. 2015;9:9731–9740. doi: 10.1021/acsnano.5b02471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin H., Xiong G., Guo S., Xu C., Xu R., Guo P., Shu D. Delivery of Anti-miRNA for Triple-Negative Breast Cancer Therapy Using RNA Nanoparticles _targeting Stem Cell Marker CD133. Mol. Ther. 2019;27:1252–1261. doi: 10.1016/j.ymthe.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun X., Xu H., Huang T., Zhang C., Wu J., Luo S. Simultaneous delivery of anti-miRNA and docetaxel with supramolecular self-assembled “chitosome” for improving chemosensitivity of triple negative breast cancer cells. Drug Deliv. Transl. Res. 2020;11:192–204. doi: 10.1007/s13346-020-00779-4. [DOI] [PubMed] [Google Scholar]
- 51.Raniolo S., Unida V., Vindigni G., Stolfi C., Iacovelli F., Desideri A., Biocca S. Combined and selective miR-21 silencing and doxorubicin delivery in cancer cells using tailored DNA nanostructures. Cell Death Dis. 2021;12:7. doi: 10.1038/s41419-020-03339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bautista-Sánchez D., Arriaga-Canon C., Pedroza-Torres A., De La Rosa-Velázquez I.A., González-Barrios R., Contreras-Espinosa L., Montiel-Manríquez R., Castro-Hernández C., Fragoso-Ontiveros V., Álvarez-Gómez R.M., et al. The Promising Role of miR-21 as a Cancer Biomarker and Its Importance in RNA-Based Therapeutics. Mol. Ther.—Nucleic Acids. 2020;20:409–420. doi: 10.1016/j.omtn.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhim J., Baek W., Seo Y., Kim J.H. From Molecular Mechanisms to Therapeutics: Understanding MicroRNA-21 in Cancer. Cells. 2022;11:2791. doi: 10.3390/cells11182791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y., Duo Y., Bi J., Zeng X., Mei L., Bao S., He L., Shan A., Zhang Y., Yu X. _targeted delivery of anti-miR-155 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Int. J. Nanomed. 2018;13:1241–1256. doi: 10.2147/IJN.S158290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma S., Rajendran V., Kulshreshtha R., Ghosh P.C. Enhanced efficacy of anti-miR-191 delivery through stearylamine liposome formulation for the treatment of breast cancer cells. Int. J. Pharm. 2017;530:387–400. doi: 10.1016/j.ijpharm.2017.07.079. [DOI] [PubMed] [Google Scholar]
- 56.Ru P., Steele R., Hsueh E.C., Ray R.B. Anti-miR-203 Upregulates SOCS3 Expression in Breast Cancer Cells and Enhances Cisplatin Chemosensitivity. Genes Cancer. 2011;2:720–727. doi: 10.1177/1947601911425832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X., Zhang H., Bai M., Ning T., Ge S., Deng T., Liu R., Zhang L., Ying G., Ba Y. Exosomes Serve as Nanoparticles to Deliver Anti-miR-214 to Reverse Chemoresistance to Cisplatin in Gastric Cancer. Mol. Ther. 2018;26:774–783. doi: 10.1016/j.ymthe.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han S., Li G., Jia M., Zhao Y., He C., Huang M., Jiang L., Wu M., Yang J., Ji X., et al. Delivery of Anti-miRNA-221 for Colorectal Carcinoma Therapy Using Modified Cord Blood Mesenchymal Stem Cells-Derived Exosomes. Front. Mol. Biosci. 2021;8:743013. doi: 10.3389/fmolb.2021.743013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dias N., Stein C.A. Antisense Oligonucleotides: Basic Concepts and Mechanisms. Mol. Cancer Ther. 2002;1:347–355. [PubMed] [Google Scholar]
- 60.Liang X.-H., Sun H., Nichols J.G., Crooke S.T. RNase H1-Dependent Antisense Oligonucleotides Are Robustly Active in Directing RNA Cleavage in Both the Cytoplasm and the Nucleus. Mol. Ther. 2017;25:2075–2092. doi: 10.1016/j.ymthe.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoo B.H., Bochkareva E., Bochkarev A., Mou T.-C., Gray D.M. 2’-O-methyl-modified phosphorothioate antisense oligonucleotides have reduced non-specific effects in vitro. Nucleic Acids Res. 2004;32:2008–2016. doi: 10.1093/nar/gkh516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prakash T.P., Kawasaki A.M., Wancewicz E.V., Shen L., Monia B.P., Ross B.S., Bhat B., Manoharan M. Comparing In Vitro and In Vivo Activity of 2′-O-[2-(Methylamino)-2-oxoethyl]- and 2′-O-Methoxyethyl-Modified Antisense Oligonucleotides. J. Med. Chem. 2008;51:2766–2776. doi: 10.1021/jm701537z. [DOI] [PubMed] [Google Scholar]
- 63.Shen X., Corey D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2017;46:1584–1600. doi: 10.1093/nar/gkx1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gagliardi M., Ashizawa A.T. Making Sense of Antisense Oligonucleotide Therapeutics _targeting Bcl-2. Pharmaceutics. 2022;14:97. doi: 10.3390/pharmaceutics14010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y., Ye W., Zhou C., Li Y., He J. Effect of polyamide nanoparticles on apoptosis of colon cancer cells induced by survivin antisense oligonucleotide. Mater. Express. 2020;10:1573–1580. doi: 10.1166/mex.2020.1769. [DOI] [Google Scholar]
- 66.Madanayake T.W., Welsh E.A., Darville L.N.F., Koomen J.M., Chalfant C.E., Haura E.B., Robinson T.J. Inhibition of Epidermal Growth Factor Receptor Signaling by Antisense Oligonucleotides as a Novel Approach to Epidermal Growth Factor Receptor Inhibition. Nucleic Acid Ther. 2022;32:391–400. doi: 10.1089/nat.2021.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Q.-Y., Ding W., Mo J.-S., Ou-Yang S.-M., Lin Z.-Y., Peng K.-R., Liu G.-P., Lu J.-J., Yue P.-B., Lei J.-P., et al. Novel STAT3 oligonucleotide compounds suppress tumor growth and overcome the acquired resistance to sorafenib in hepatocellular carcinoma. Acta Pharmacol. Sin. 2024;45:1701–1714. doi: 10.1038/s41401-024-01261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandez-Rodriguez L., Cianciaruso C., Bill R., Trefny M.P., Klar R., Kirchhammer N., Buchi M., Festag J., Michel S., Kohler R.H., et al. Dual TLR9 and PD-L1 _targeting unleashes dendritic cells to induce durable antitumor immunity. J. ImmunoTherapy Cancer. 2023;11:e006714. doi: 10.1136/jitc-2023-006714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang X.-H., Vickers T.A., Guo S., Crooke S.T. Efficient and specific knockdown of small non-coding RNAs in mammalian cells and in mice. Nucleic Acids Res. 2010;39:e13. doi: 10.1093/nar/gkq1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vickers T.A., Crooke S.T. Antisense Oligonucleotides Capable of Promoting Specific _target mRNA Reduction via Competing RNase H1-Dependent and Independent Mechanisms. PLoS ONE. 2014;9:e108625. doi: 10.1371/journal.pone.0108625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang K.C., Chang H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iyer M.K., Niknafs Y.S., Malik R., Singhal U., Sahu A., Hosono Y., Barrette T.R., Prensner J.R., Evans J.R., Zhao S., et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smolarz B., Zadrożna-Nowak A., Romanowicz H. The Role of lncRNA in the Development of Tumors, including Breast Cancer. Int. J. Mol. Sci. 2021;22:8427. doi: 10.3390/ijms22168427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang M., Lu H., Liu J., Wu S., Kim P., Zhou X. lncRNAfunc: A knowledgebase of lncRNA function in human cancer. Nucleic Acids Res. 2021;50:D1295–D1306. doi: 10.1093/nar/gkab1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang M.-C., Ni J.-J., Cui W.-Y., Wang B.-Y., Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 2019;9:1354–1366. [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou T., Kim Y., MacLeod A.R. _targeting Long Noncoding RNA with Antisense Oligonucleotide Technology as Cancer Therapeutics. Methods Mol. Biol. 2016;1402:199–213. doi: 10.1007/978-1-4939-3378-5_16. [DOI] [PubMed] [Google Scholar]
- 77.Singh N.N., Luo D., Singh R.N. Methods in Molecular Biology. Humana Press; New York, NY, USA: 2018. Pre-mRNA Splicing Modulation by Antisense Oligonucleotides; pp. 415–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bauman J., Jearawiriyapaisarn N., Kole R. Therapeutic Potential of Splice-Switching Oligonucleotides. Oligonucleotides. 2009;19:1–13. doi: 10.1089/oli.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang X.-H., Shen W., Sun H., Migawa M.T., Vickers T.A., Crooke S.T. Translation efficiency of mRNAs is increased by antisense oligonucleotides _targeting upstream open reading frames. Nat. Biotechnol. 2016;34:875–880. doi: 10.1038/nbt.3589. [DOI] [PubMed] [Google Scholar]
- 80.Lim K.H., Han Z., Jeon H.Y., Kach J., Jing E., Weyn-Vanhentenryck S., Downs M., Corrionero A., Oh R., Scharner J., et al. Antisense oligonucleotide modulation of non-productive alternative splicing upregulates gene expression. Nat. Commun. 2020;11:3501. doi: 10.1038/s41467-020-17093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ward W.L., Plakos K., DeRose V.J. Nucleic Acid Catalysis: Metals, Nucleobases, and Other Cofactors. Chem. Rev. 2014;114:4318–4342. doi: 10.1021/cr400476k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma L., Liu J. Catalytic Nucleic Acids: Biochemistry, Chemical Biology, Biosensors, and Nanotechnology. iScience. 2020;23:100815. doi: 10.1016/j.isci.2019.100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang F., Saran R., Liu J. Tandem DNAzymes for mRNA cleavage: Choice of enzyme, metal ions and the antisense effect. Bioorg. Med. Chem. Lett. 2015;25:1460–1463. doi: 10.1016/j.bmcl.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 84.Kashani-Sabet M. Ribozyme Therapeutics. J. Investig. Dermatol. Symp. Proc. 2002;7:76–78. doi: 10.1046/j.1523-1747.2002.19642.x. [DOI] [PubMed] [Google Scholar]
- 85.Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 86.Kruger K., Grabowski P.J., Zaug A.J., Sands J., Gottschling D.E., Cech T.R. Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of tetrahymena. Cell. 1982;31:147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- 87.Breaker R.R., Joyce G.F. A DNA enzyme that cleaves RNA. Chem. Biol. 1994;1:223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 88.Wilson D.S., Szostak J.W. In Vitro Selection of Functional Nucleic Acids. Annu. Rev. Biochem. 1999;68:611–647. doi: 10.1146/annurev.biochem.68.1.611. [DOI] [PubMed] [Google Scholar]
- 89.Silverman S.K. Deoxyribozymes: Selection Design and Serendipity in the Development of DNA Catalysts. Acc. Chem. Res. 2009;42:1521–1531. doi: 10.1021/ar900052y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scott W.G., Horan L.H., Martick M. The Hammerhead Ribozyme: Structure, Catalysis, and Gene Regulation. Prog. Mol. Biol. Transl. Sci. 2013;120:1–23. doi: 10.1016/B978-0-12-381286-5.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puerta-Fernández E., Romero-López C., Barroso-delJesus A., Berzal-Herranz A. Ribozymes: Recent advances in the development of RNA tools. FEMS Microbiol. Rev. 2003;27:75–97. doi: 10.1016/S0168-6445(03)00020-2. [DOI] [PubMed] [Google Scholar]
- 92.Tokunaga T., Tsuchida T., Kijima H., Okamoto K., Oshika Y., Sawa N., Ohnishi Y., Yamazaki H., Miura S., Ueyama Y., et al. Ribozyme-mediated inactivation of mutant K-ras oncogene in a colon cancer cell line. Br. J. Cancer. 2000;83:833–839. doi: 10.1054/bjoc.2000.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aigner A., Renneberg H., Bojunga J., Apel J., Nelson P.S., Czubayko F. Ribozyme-_targeting of a secreted FGF-binding protein (FGF-BP) inhibits proliferation of prostate cancer cells in vitro and in vivo. Oncogene. 2002;21:5733–5742. doi: 10.1038/sj.onc.1205560. [DOI] [PubMed] [Google Scholar]
- 94.Nagata J., Kijima H., Hatanaka H., Asai S., Miyachi H., Takagi A., Miwa T., Mine T., Yamazaki H., Nakamura M., et al. Reversal of Cisplatin and Multidrug Resistance by Ribozyme-Mediated Glutathione Suppression. Biochem. Biophys. Res. Commun. 2001;286:406–413. doi: 10.1006/bbrc.2001.5399. [DOI] [PubMed] [Google Scholar]
- 95.Gao P., Zhou G.-Y., Guo L.-L., Zhang Q.-H., Zhen J.-H., Fang A.-J., Lin X.-Y. Reversal of drug resistance in breast carcinoma cells by anti-mdr1 ribozyme regulated by the tumor-specific MUC-1 promoter. Cancer Lett. 2007;256:81–89. doi: 10.1016/j.canlet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 96.Han S.R., Lee C.H., Im J.Y., Kim J.H., Kim J.H., Kim S.J., Cho Y.W., Kim E., Kim Y., Ryu J.-H., et al. _targeted suicide gene therapy for liver cancer based on ribozyme-mediated RNA replacement through post-transcriptional regulation. Mol. Ther.—Nucleic Acids. 2021;23:154–168. doi: 10.1016/j.omtn.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang X., Wang M., Liu Y., Gui Y. Synthesis of RNA-based gene regulatory devices for redirecting cellular signaling events mediated by p53. Theranostics. 2021;11:4688–4698. doi: 10.7150/thno.55856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huo W., Li X., Wang B., Zhang H., Zhang J., Yang X., Jin Y. Recent advances of DNAzyme-based nanotherapeutic platform in cancer gene therapy. Biophys. Rep. 2020;6:256–265. doi: 10.1007/s41048-020-00123-w. [DOI] [Google Scholar]
- 99.Li J., Zheng W., Kwon A.H., Lu Y. In vitro selection and characterization of a highly efficient Zn(II)-dependent RNA-cleaving deoxyribozyme. Nucleic Acids Res. 2000;28:481–488. doi: 10.1093/nar/28.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santoro S.W., Joyce G.F. A general purpose RNA-cleaving DNA enzyme. Proc. Natl. Acad. Sci. USA. 1997;94:4262. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khachigian L.M. DNAzymes: Cutting a path to a new class of therapeutics. Curr. Opin. Mol. Ther. 2002;4:119–121. [PubMed] [Google Scholar]
- 102.Cairns M.J., King A., Sun L.Q. Optimisation of the 10-23 DNAzyme-substrate pairing interactions enhanced RNA cleavage activity at purine-cytosine _target sites. Nucleic Acids Res. 2003;31:2883–2889. doi: 10.1093/nar/gkg378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang X., Li Z., Zhang L., He J., Sun L.-Q. Selection and antitumor activity of anti-Bcl-2 DNAzymes. Biochem. Biophys. Res. Commun. 2016;479:544–550. doi: 10.1016/j.bbrc.2016.09.107. [DOI] [PubMed] [Google Scholar]
- 104.Zhang M., Sun Y.-F., Luo S. Ani-survivin DNAzymes Inhibit Cell Proliferation and Migration in Breast Cancer Cell Line MCF-7. Asian Pac. J. Cancer Prev. 2012;13:6233–6237. doi: 10.7314/APJCP.2012.13.12.6233. [DOI] [PubMed] [Google Scholar]
- 105.Wang Z., Niu J., Zhao C., Wang X., Ren J., Qu X. A Bimetallic Metal–Organic Framework Encapsulated with DNAzyme for Intracellular Drug Synthesis and Self-Sufficient Gene Therapy. Angew. Chem. 2021;133:12539–12545. doi: 10.1002/ange.202016442. [DOI] [PubMed] [Google Scholar]
- 106.De Bock C.E., Lin Z., Itoh T., Morris D., Murrell G., Wang Y. Inhibition of urokinase receptor gene expression and cell invasion by anti-uPAR DNAzymes in osteosarcoma cells. FEBS J. 2005;272:3572–3582. doi: 10.1111/j.1742-4658.2005.04778.x. [DOI] [PubMed] [Google Scholar]
- 107.Du S., Chen C., Qu S., Song H., Yang J., Li Y., Liu K., Lu Q., Luo W., Wang R., et al. DNAzyme-Assisted Nano-Herb Delivery System for Multiple Tumor Immune Activation. Small. 2022;18:2203942. doi: 10.1002/smll.202203942. [DOI] [PubMed] [Google Scholar]
- 108.Cai H., Santiago F.S., Prado-Lourenco L., Wang B., Patrikakis M., Davenport M.P., Maghzal G.J., Stocker R., Parish C.R., Chong B.H., et al. DNAzyme _targeting c- jun Suppresses Skin Cancer Growth. Sci. Transl. Med. 2012;4:139ra82. doi: 10.1126/scitranslmed.3003960. [DOI] [PubMed] [Google Scholar]
- 109.Zhang M., Drummen G.P.C., Luo S. Anti-insulin-like growth factor-IIP3 DNAzymes inhibit cell proliferation and induce caspase-dependent apoptosis in human hepatocarcinoma cell lines. Drug Des. Dev. Ther. 2013;7:1089–1102. doi: 10.2147/DDDT.S48971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schubert S. RNA Cleaving “10–23” DNAzymes with Enhanced Stability and Activity. Nucleic Acids Res. 2003;31:5982–5992. doi: 10.1093/nar/gkg791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vester B., Lundberg L.B., Sørensen M.D., Babu B.R., Douthwaite S., Wengel J. LNAzymes: Incorporation of LNA-Type Monomers into DNAzymes Markedly Increases RNA Cleavage. J. Am. Chem. Soc. 2002;124:13682–13683. doi: 10.1021/ja0276220. [DOI] [PubMed] [Google Scholar]
- 112.Thai H.B.D., Levi-Acobas F., Yum S.-Y., Jang G., Hollenstein M., Ahn D.-R. Tetrahedral DNAzymes for enhanced intracellular gene-silencing activity. Chem. Commun. 2018;54:9410–9413. doi: 10.1039/C8CC05721D. [DOI] [PubMed] [Google Scholar]
- 113.Lee M., Kim M., Lee M., Kim S., Park N. Nanosized DNA Hydrogel Functionalized with a DNAzyme Tetrahedron for Highly Efficient Gene Silencing. Biomacromolecules. 2024;25:4913–4924. doi: 10.1021/acs.biomac.4c00356. [DOI] [PubMed] [Google Scholar]
- 114.Ren L., Chen X., Feng C., Ding L., Liu X., Chen T., Zhang F., Li Y., Ma Z., Tian B., et al. Visualized and cascade-enhanced gene silencing by smart DNAzyme-graphene nanocomplex. Nano Res. 2020;13:2165–2174. doi: 10.1007/s12274-020-2826-5. [DOI] [Google Scholar]
- 115.He M., He M., Nie C., Yi J., Zhang J., Chen T., Chu X. mRNA-Activated Multifunctional DNAzyme Nanotweezer for Intracellular mRNA Sensing and Gene Therapy. ACS Appl. Mater. Interfaces. 2021;13:8015–8025. doi: 10.1021/acsami.0c21601. [DOI] [PubMed] [Google Scholar]
- 116.Hermann T., Patel D.J. Adaptive Recognition by Nucleic Acid Aptamers. Science. 2000;287:820–825. doi: 10.1126/science.287.5454.820. [DOI] [PubMed] [Google Scholar]
- 117.Hayashi T., Oshima H., Mashima T., Nagata T., Katahira M., Kinoshita M. Binding of an RNA aptamer and a partial peptide of a prion protein: Crucial importance of water entropy in molecular recognition. Nucleic Acids Res. 2014;42:6861–6875. doi: 10.1093/nar/gku382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Partha R., Kristi D.V., Erin E.S., Rebecca S. Application of aptamers for _targeted theraputics. Arch. Immunol. Ther. Exp. 2013;61:255–271. doi: 10.1007/s00005-013-0227-0. [DOI] [PubMed] [Google Scholar]
- 119.Dunn M.R., Jimenez R.M., Chaput J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017;1:0076. doi: 10.1038/s41570-017-0076. [DOI] [Google Scholar]
- 120.Zhou J., Rossi J.J. Cell-type-specific, Aptamer-functionalized Agents for _targeted Disease Therapy. Mol. Ther.—Nucleic Acids. 2014;3:e169. doi: 10.1038/mtna.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ellington A., Szostak J. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 122.Bock L.C., Griffin L.C., Latham J.A., Vermaas E.H., Toole J.J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 1992;355:564–566. doi: 10.1038/355564a0. [DOI] [PubMed] [Google Scholar]
- 123.Ni S., Zhuo Z., Pan Y., Yu Y., Li F., Liu J., Wang L., Wu X., Li D., Wan Y., et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces. 2021;13:9500–9519. doi: 10.1021/acsami.0c05750. [DOI] [PubMed] [Google Scholar]
- 124.Dua P., Kim S., Lee D.-K. Nucleic acid aptamers _targeting cell-surface proteins. Methods. 2011;54:215–225. doi: 10.1016/j.ymeth.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 125.Kim D.-H., Seo J.-M., Shin K.-J., Yang S.-G. Design and clinical developments of aptamer-drug conjugates for _targeted cancer therapy. Biomater. Res. 2021;25:42. doi: 10.1186/s40824-021-00244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Poolsup S., Kim C.-Y. Therapeutic applications of synthetic nucleic acid aptamers. Curr. Opin. Biotechnol. 2017;48:180–186. doi: 10.1016/j.copbio.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 127.Vu C.Q., Rotkrua P., Soontornworajit B., Tantirungrotechai Y. Effect of PDGF-B aptamer on PDGFRβ/PDGF-B interaction: Molecular dynamics study. J. Mol. Graph. Model. 2018;82:145–156. doi: 10.1016/j.jmgm.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 128.Sae-Lim S., Soontornworajit B., Rotkrua P. Inhibition of Colorectal Cancer Cell Proliferation by Regulating Platelet-Derived Growth Factor B Signaling with a DNA Aptamer. Asian Pac. J. Cancer Prev. 2019;20:487–494. doi: 10.31557/APJCP.2019.20.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu S.-C., Alomran R., Chernikova S.B., Lartey F., Stafford J., Jang T., Merchant M., Zboralski D., Zöllner S., Kruschinski A., et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro-Oncol. 2013;16:21–28. doi: 10.1093/neuonc/not149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zboralski D., Hoehlig K., Eulberg D., Frömming A., Vater A. Increasing Tumor-Infiltrating T Cells through Inhibition of CXCL12 with NOX-A12 Synergizes with PD-1 Blockade. Cancer Immunol. Res. 2017;5:950–956. doi: 10.1158/2326-6066.CIR-16-0303. [DOI] [PubMed] [Google Scholar]
- 131.Hoellenriegel J., Zboralski D., Maasch C., Rosin N.Y., Wierda W.G., Keating M.J., Kruschinski A., Burger J.A. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood. 2014;123:1032–1039. doi: 10.1182/blood-2013-03-493924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gijs M., Penner G., Blackler G., Impens N., Baatout S., Luxen A., Aerts A. Improved Aptamers for the Diagnosis and Potential Treatment of HER2-Positive Cancer. Pharmaceuticals. 2016;9:29. doi: 10.3390/ph9020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mahlknecht G., Maron R., Mancini M., Schechter B., Sela M., Yarden Y. Aptamer to ErbB-2/HER2 enhances degradation of the _target and inhibits tumorigenic growth. Proc. Natl. Acad. Sci. USA. 2013;110:8170–8175. doi: 10.1073/pnas.1302594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guo L., Zhang Y., Wang Y., Xie M., Dai J., Qu Z., Zhou M., Cao S., Shi J., Wang L., et al. Directing Multivalent Aptamer-Receptor Binding on the Cell Surface with Programmable Atom-Like Nanoparticles. Angew. Chem. Int. Ed. 2022;61:e202117168. doi: 10.1002/anie.202117168. [DOI] [PubMed] [Google Scholar]
- 135.Zhou H., Jiao P., Yang L., Li X., Yan B. Enhancing Cell Recognition by Scrutinizing Cell Surfaces with a Nanoparticle Array. J. Am. Chem. Soc. 2010;133:680–682. doi: 10.1021/ja108527y. [DOI] [PubMed] [Google Scholar]
- 136.Chen S., Xu Z., Yang W., Lin X., Li J., Li J., Yang H. Logic-Gate-Actuated DNA-Controlled Receptor Assembly for the Programmable Modulation of Cellular Signal Transduction. Angew. Chem. 2019;131:18354–18358. doi: 10.1002/ange.201908971. [DOI] [PubMed] [Google Scholar]
- 137.Wang L., Liang H., Sun J., Liu Y., Li J., Li J., Li J., Yang H. Bispecific Aptamer Induced Artificial Protein-Pairing: A Strategy for Selective Inhibition of Receptor Function. J. Am. Chem. Soc. 2019;141:12673–12681. doi: 10.1021/jacs.9b05123. [DOI] [PubMed] [Google Scholar]
- 138.Miao Y., Gao Q., Mao M., Zhang C., Yang L., Yang Y., Han D. Bispecific Aptamer Chimeras Enable _targeted Protein Degradation on Cell Membranes. Angew. Chem. 2021;133:11367–11371. doi: 10.1002/ange.202102170. [DOI] [PubMed] [Google Scholar]
- 139.Hoshiyama J., Okada Y., Cho S., Ueki R., Sando S. Apt-clean: Aptamer-mediated cleavage of extracellular antigens for the inhibition of membrane protein functions. Biomater. Sci. 2023;11:445–449. doi: 10.1039/D2BM01695H. [DOI] [PubMed] [Google Scholar]
- 140.Varadé J., Magadán S., González-Fernández Á. Human immunology and immunotherapy: Main achievements and challenges. Cell. Mol. Immunol. 2020;18:805–828. doi: 10.1038/s41423-020-00530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Swann J.B., Smyth M.J. Immune surveillance of tumors. J. Clin. Investig. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim R., Emi M., Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kejamurthy P., Devi K.T.R. Immune checkpoint inhibitors and cancer immunotherapy by aptamers: An overview. Med. Oncol. 2023;41:40. doi: 10.1007/s12032-023-02267-4. [DOI] [PubMed] [Google Scholar]
- 145.Prodeus A., Abdul-Wahid A., Fischer N.W., Huang E.H.B., Cydzik M., Gariépy J. _targeting the PD-1/PD-L1 immune evasion axis with DNA aptamers as a novel therapeutic strategy for the treatment of disseminated cancers. Mol. Ther. Nucleic Acids. 2015;4:e237. doi: 10.1038/mtna.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang D., Zheng Y., Lin Z., Liu X., Li J., Yang H., Tan W. Equipping Natural Killer Cells with Specific _targeting and Checkpoint Blocking Aptamers for Enhanced Adoptive Immunotherapy in Solid Tumors. Angew. Chem. 2020;132:12120–12126. doi: 10.1002/ange.202002145. [DOI] [PubMed] [Google Scholar]
- 147.Huang B.T., Lai W.Y., Chang Y.C., Wang J.W., Yeh S.D., Lin E.P.Y., Yang P.C. A CTLA-4 Antagonizing DNA Aptamer with Antitumor Effect. Mol. Ther.—Nucleic Acids. 2017;8:520–528. doi: 10.1016/j.omtn.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boltz A., Piater B., Toleikis L., Guenther R., Kolmar H., Hock B. Bi-specific Aptamers Mediating Tumor Cell Lysis. J. Biol. Chem. 2011;286:21896–21905. doi: 10.1074/jbc.M111.238261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang Y., Sun X., Xu J., Cui C., Yazd H.S., Pan X., Zhu Y., Chen X., Li X., Li J., et al. Circular Bispecific Aptamer-Mediated Artificial Intercellular Recognition for _targeted T Cell Immunotherapy. ACS Nano. 2020;14:9562–9571. doi: 10.1021/acsnano.9b09884. [DOI] [PubMed] [Google Scholar]
- 150.Zheng A., Du Y., Wang Y., Zheng Y., Ning Z., Wu M., Zhang C., Zhang D., Liu J., Liu X. CD16/PD-L1 bi-specific aptamer for cancer immunotherapy through recruiting NK cells and acting as immunocheckpoint blockade. Mol. Ther.—Nucleic Acids. 2022;27:998–1009. doi: 10.1016/j.omtn.2022.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thomas B.J., Porciani D., Burke D.H. Cancer immunomodulation using bispecific aptamers. Mol. Ther.—Nucleic Acids. 2022;27:894–915. doi: 10.1016/j.omtn.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mahmoudian F., Ahmari A., Shabani S., Sadeghi B., Fahimirad S., Fattahi F. Aptamers as an approach to _targeted cancer therapy. Cancer Cell Int. 2024;24:108. doi: 10.1186/s12935-024-03295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kroschinsky F., Stölzel F., Von Bonin S., Beutel G., Kochanek M., Kiehl M., Schellongowski P. New drugs, new toxicities: Severe side effects of modern _targeted and immunotherapy of cancer and their management. Crit. Care. 2017;21:89. doi: 10.1186/s13054-017-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lo C.W.-S., Chan C.K.W., Yu J., He M., Choi C.H.J., Lau J.Y.W., Wong N. Development of CD44E/s dual-_targeting DNA aptamer as nanoprobe to deliver treatment in hepatocellular carcinoma. Nanotheranostics. 2022;6:161–174. doi: 10.7150/ntno.62639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jeong H.Y., Kim H., Lee M., Hong J., Lee J.H., Kim J., Choi M.J., Park Y.S., Kim S.-C. Development of HER2-Specific Aptamer-Drug Conjugate for Breast Cancer Therapy. Int. J. Mol. Sci. 2020;21:9764. doi: 10.3390/ijms21249764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kciuk M., Gielecińska A., Mujwar S., Kołat D., Kałuzińska-Kołat Ż., Celik I., Kontek R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells. 2023;12:659. doi: 10.3390/cells12040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yao F., An Y., Li X., Li Z., Duan J., Yang X.-D. _targeted Therapy of Colon Cancer by Aptamer-Guided Holliday Junctions Loaded with Doxorubicin. Int. J. Nanomed. 2020;15:2119–2129. doi: 10.2147/IJN.S240083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tan Y., Li Y., Qu Y.-X., Su Y., Peng Y., Zhao Z., Fu T., Wang X.-Q., Tan W. Aptamer-Peptide Conjugates as _targeted Chemosensitizers for Breast Cancer Treatment. ACS Appl. Mater. Interfaces. 2020;13:9436–9444. doi: 10.1021/acsami.0c18282. [DOI] [PubMed] [Google Scholar]
- 159.Omer M., Andersen V.L., Nielsen J.S., Wengel J., Kjems J. Improved Cancer _targeting by Multimerizing Aptamers on Nanoscaffolds. Mol. Ther.—Nucleic Acids. 2020;22:994–1003. doi: 10.1016/j.omtn.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Alves L.N., Missailidis S., Lage C.A., De Almeida C.E.B. Anti-MUC1 Aptamer as Carrier Tool of the Potential Radiosensitizer 1,10 Phenanthroline in MCF-7 Breast Cancer Cells. Anticancer Res. 2019;39:1859–1867. doi: 10.21873/anticanres.13293. [DOI] [PubMed] [Google Scholar]
- 161.Ghahremani F., Kefayat A., Shahbazi-Gahrouei D., Motaghi H., Mehrgardi M.A., Haghjooy-Javanmard S. AS1411 aptamer-_targeted gold nanoclusters effect on the enhancement of radiation therapy efficacy in breast tumor-bearing mice. Nanomedicine. 2018;13:2563–2578. doi: 10.2217/nnm-2018-0180. [DOI] [PubMed] [Google Scholar]
- 162.Dassie J.P., Liu X.-Y., Thomas G.S., Whitaker R.M., Thiel K.W., Stockdale K.R., Meyerholz D.K., McCaffrey A.P., McNamara J.O., Giangrande P.H. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat. Biotechnol. 2009;27:839–846. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang S., Ding J., Zhou W. An aptamer-tethered, DNAzyme-embedded molecular beacon for simultaneous detection and regulation of tumor-related genes in living cells. Analyst. 2019;144:5098–5107. doi: 10.1039/C9AN01097A. [DOI] [PubMed] [Google Scholar]
- 164.Liu H.Y., Yu X., Liu H., Wu D., She J.-X. Co-_targeting EGFR and survivin with a bivalent aptamer-dual siRNA chimera effectively suppresses prostate cancer. Sci. Rep. 2016;6:30346. doi: 10.1038/srep30346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hong S., Sun N., Liu M., Wang J., Pei R. Building a chimera of aptamer–antisense oligonucleotide for silencing galectin-1 gene. RSC Adv. 2016;6:112445–112450. doi: 10.1039/C6RA21250F. [DOI] [Google Scholar]
- 166.Garneau J.E., Dupuis M.È., Villion M., Romero D.A., Barrangou R., Boyaval P., Fremaux C., Horvath P., Magadán A.H., Moineau S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- 167.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gaj T., Gersbach C.A., Barbas C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Martinez-Lage M., Puig-Serra P., Menendez P., Torres-Ruiz R., Rodriguez-Perales S. CRISPR/Cas9 for Cancer Therapy: Hopes and Challenges. Biomedicines. 2018;6:105. doi: 10.3390/biomedicines6040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu C., Zhang L., Liu H., Cheng K. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J. Control. Release. 2017;266:17–26. doi: 10.1016/j.jconrel.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen H., Choi J., Bailey S. Cut Site Selection by the Two Nuclease Domains of the Cas9 RNA-guided Endonuclease. J. Biol. Chem. 2014;289:13284–13294. doi: 10.1074/jbc.M113.539726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stefanoudakis D., Kathuria-Prakash N., Sun A.W., Abel M., Drolen C.E., Ashbaugh C., Zhang S., Hui G., Tabatabaei Y.A., Zektser Y., et al. The Potential Revolution of Cancer Treatment with CRISPR Technology. Cancers. 2023;15:1813. doi: 10.3390/cancers15061813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gilbert L.A., Larson M.H., Morsut L., Liu Z., Brar G.A., Torres S.E., Stern-Ginossar N., Brandman O., Whitehead E.H., Doudna J.A., et al. CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dominguez A.A., Lim W.A., Qi L.S. Beyond editing: Repurposing CRISPR–Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 2015;17:5–15. doi: 10.1038/nrm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Qi Y., Liu Y., Yu B., Hu Y., Zhang N., Zheng Y., Yang M., Xu F. A Lactose-Derived CRISPR/Cas9 Delivery System for Efficient Genome Editing In Vivo to Treat Orthotopic Hepatocellular Carcinoma. Adv. Sci. 2020;7:2001424. doi: 10.1002/advs.202001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.He Z.-Y., Zhang Y.-G., Yang Y.-H., Ma C.-C., Wang P., Du W., Li L., Xiang R., Song X.-R., Zhao X., et al. In Vivo Ovarian Cancer Gene Therapy Using CRISPR-Cas9. Hum. Gene Ther. 2018;29:223–233. doi: 10.1089/hum.2017.209. [DOI] [PubMed] [Google Scholar]
- 177.Koo T., Yoon A.-R., Cho H.-Y., Bae S., Yun C.-O., Kim J.-S. Selective disruption of an oncogenic mutant allele by CRISPR/Cas9 induces efficient tumor regression. Nucleic Acids Res. 2017;45:7897–7908. doi: 10.1093/nar/gkx490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hussen B.M., Rasul M.F., Abdullah S.R., Hidayat H.J., Faraj G.S.H., Ali F.A., Salihi A., Baniahmad A., Ghafouri-Fard S., Rahman M., et al. _targeting miRNA by CRISPR/Cas in cancer: Advantages and challenges. Mil. Med. Res. 2023;10:32. doi: 10.1186/s40779-023-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yoshino H., Yonemori M., Miyamoto K., Tatarano S., Kofuji S., Nohata N., Nakagawa M., Enokida H. microRNA-210-3p depletion by CRISPR/Cas9 promoted tumorigenesis through revival of TWIST1 in renal cell carcinoma. Onco_target. 2017;8:20881–20894. doi: 10.18632/onco_target.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.De Santa-Inez D.C., Fuziwara C.S., Saito K.C., Kimura E.T. _targeting the Highly Expressed microRNA miR-146b with CRISPR/Cas9n Gene Editing System in Thyroid Cancer. Int. J. Mol. Sci. 2021;22:7992. doi: 10.3390/ijms22157992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jiang F., Liang Y., Wei W., Zou C., Chen G., Wan Y., Liu Z., Yang Y., Han Z., Zhu J., et al. Functional classification of prostate cancer-associated miRNAs through CRISPR/Cas9-mediated gene knockout. Mol. Med. Rep. 2020;5:3777–3784. doi: 10.3892/mmr.2020.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vaghari-Tabari M., Hassanpour P., Sadeghsoltani F., Malakoti F., Alemi F., Qujeq D., Asemi Z., Yousefi B. CRISPR/Cas9 gene editing: A new approach for overcoming drug resistance in cancer. Cell. Mol. Biol. Lett. 2022;27:49. doi: 10.1186/s11658-022-00348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li T., Yang Y., Qi H., Cui W., Zhang L., Fu X., He X., Liu M., Li P.-F., Yu T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. _target. Ther. 2023;8:36. doi: 10.1038/s41392-023-01309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ansori A.N., Antonius Y., Susilo R.J., Hayaza S., Kharisma V.D., Parikesit A.A., Zainul R.J., Jakhmola V., Saklani T., Rebezov M., et al. Application of CRISPR-Cas9 genome editing technology in various fields: A review. Narra J. 2023;3:e184. doi: 10.52225/narra.v3i2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Guo C., Ma X., Gao F., Guo Y. Off-_target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023;11:1143157. doi: 10.3389/fbioe.2023.1143157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bai H., Lester G.M.S., Petishnok L.C., Dean D.A. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci. Rep. 2017;37:BSR20160616. doi: 10.1042/BSR20160616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Martínez-Puente D.H., Pérez-Trujillo J.J., Zavala-Flores L.M., García-García A., Villanueva-Olivo A., Rodríguez-Rocha H., Valdés J., Saucedo-Cárdenas O., De Oca-Luna R.M., De Jesús Loera-Arias M. Plasmid DNA for Therapeutic Applications in Cancer. Pharmaceutics. 2022;14:1861. doi: 10.3390/pharmaceutics14091861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu N. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines. 2019;7:37. doi: 10.3390/vaccines7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gómez-Navarro J., Arafat W., Xiang J. Gene therapy for carcinoma of the breast: Pro-apoptotic gene therapy. Breast Cancer Res. 1999;2:32. doi: 10.1186/bcr27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Neves A.R., Sousa A., Faria R., Albuquerque T., Queiroz J.A., Costa D. Cancer gene therapy mediated by RALA/plasmid DNA vectors: Nitrogen to phosphate groups ratio (N/P) as a tool for tunable transfection efficiency and apoptosis. Colloids Surf. B Biointerfaces. 2020;185:110610. doi: 10.1016/j.colsurfb.2019.110610. [DOI] [PubMed] [Google Scholar]
- 191.Singh S., Asal R., Bhagat S. Multifunctional antioxidant nanoliposome-mediated delivery of PTEN plasmids restore the expression of tumor suppressor protein and induce apoptosis in prostate cancer cells. J. Biomed. Mater. Res. Part A. 2018;106:3152–3164. doi: 10.1002/jbm.a.36510. [DOI] [PubMed] [Google Scholar]
- 192.Hong S.-H., Lee J.-H., Jiang H.-L., Kim J.-E., Lee A.Y., Kim S., Cho C.-S., Cho M.-H. Dual Expression of shAkt1 and Pdcd4 Suppresses Lung Tumorigenesis in K-rasLA1 Mice. Anticancer Res. 2015;35:2015–2019. [PubMed] [Google Scholar]
- 193.Zarovni N., Vago R., Soldà T., Monaco L., Fabbrini M.S. Saporin as a novel suicide gene in anticancer gene therapy. Cancer Gene Ther. 2006;14:165–173. doi: 10.1038/sj.cgt.7700998. [DOI] [PubMed] [Google Scholar]
- 194.Rodriguez B.L., Yu Z., Chung W.G., Weiss R., Cui Z. Replicase-based plasmid DNA shows anti-tumor activity. BMC Cancer. 2011;11:110. doi: 10.1186/1471-2407-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mizrahi A., Czerniak A., Levy T., Amiur S., Gallula J., Matouk I., Abu-Lail R., Sorin V., Birman T., De Groot N., et al. Development of _targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J. Transl. Med. 2009;7:69. doi: 10.1186/1479-5876-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim H., Danishmalik S.N., Hwang H., Sin J., Oh J., Cho Y., Lee H., Jeong M., Kim S., Hong H.J. Gene therapy using plasmid DNA-encoded anti-HER2 antibody for cancers that overexpress HER2. Cancer Gene Ther. 2016;23:341–347. doi: 10.1038/cgt.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Muthumani K., Marnin L., Kudchodkar S.B., Perales-Puchalt A., Choi H., Agarwal S., Scott V.L., Reuschel E.L., Zaidi F.I., Duperret E.K., et al. Novel prostate cancer immunotherapy with a DNA-encoded anti-prostate-specific membrane antigen monoclonal antibody. Cancer Immunol. Immunother. 2017;66:1577–1588. doi: 10.1007/s00262-017-2042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pandya A., Shah Y., Kothari N., Postwala H., Shah A., Parekh P., Chorawala M.R. The future of cancer immunotherapy: DNA vaccines leading the way. Med. Oncol. 2023;40:200. doi: 10.1007/s12032-023-02060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kawano H., Nishikawa M., Mitsui M., Takahashi Y., Kako K., Yamaoka K., Watanabe Y., Takakura Y. Improved anti-cancer effect of interferon gene transfer by sustained expression using CpG-reduced plasmid DNA. Int. J. Cancer. 2007;121:401–406. doi: 10.1002/ijc.22636. [DOI] [PubMed] [Google Scholar]
- 200.Pang J., Zhuang B., Zhang L.-M. A co-carrier for plasmid DNA and curcumin delivery to treat pancreatic cancer via dendritic poly(l-lysine) modified amylose. Int. J. Biol. Macromol. 2023;253:127467. doi: 10.1016/j.ijbiomac.2023.127467. [DOI] [PubMed] [Google Scholar]
- 201.Chen J., Liu H., Jehng T., Li Y., Chen Z., Lee K.-D., Shen H.-T., Jones L., Huang X.F., Chen S.-Y. A Novel Anti-PD-L1 Vaccine for Cancer Immunotherapy and Immunoprevention. Cancers. 2019;11:1909. doi: 10.3390/cancers11121909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu X., Yang Y., Zheng X., Liu M., Wang G. Enhancedanti-tumor efficacy through a combination of intramuscularly expressed DNA vaccine and plasmid-encoded PD-1 antibody. Front. Immunol. 2023;14:1169850. doi: 10.3389/fimmu.2023.1169850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Duperret E.K., Wise M.C., Trautz A., Villarreal D.O., Ferraro B., Walters J., Yan J., Khan A., Masteller E., Humeau L., et al. Synergy of Immune Checkpoint Blockade with a Novel Synthetic Consensus DNA Vaccine _targeting TERT. Mol. Ther. 2018;26:435–445. doi: 10.1016/j.ymthe.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kos S., Lopes A., Preat V., Cemazar M., Tratar U.L., Ucakar B., Vanvarenberg K., Sersa G., Vandermeulen G. Intradermal DNA vaccination combined with dual CTLA-4 and PD-1 blockade provides robust tumor immunity in murine melanoma. PLoS ONE. 2019;14:e0217762. doi: 10.1371/journal.pone.0217762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Taxman D.J., Moore C.B., Guthrie E.H., Huang M.T.-H. Methods in Molecular Biology. Humana Press; New York, NY, USA: 2010. Short Hairpin RNA (shRNA): Design, Delivery, and Assessment of Gene Knockdown; pp. 139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Künnapuu K., Veiman K.-L., Porosk L., Rammul E., Kiisholts K., Langel Ü., Kurrikoff K. Tumor gene therapy by systemic delivery of plasmid DNA with cell-penetrating peptides. FASEB BioAdvances. 2018;1:105–114. doi: 10.1096/fba.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Seraj S., Cho Y.J., Lee J.-W., Ahn H.J. Cytoplasmic expression of EGFR shRNA using a modified T7 autogene-based hybrid mRNA/DNA system induces long-term EGFR silencing and prolongs antitumor effects. Biochem. Pharmacol. 2020;171:113735. doi: 10.1016/j.bcp.2019.113735. [DOI] [PubMed] [Google Scholar]
- 208.Zhao L., Luo Y., Huang Q., Cao Z., Yang X. Photo-Enhanced CRISPR/Cas9 System Enables Robust PD-L1 Gene Disruption in Cancer Cells and Cancer Stem-Like Cells for Efficient Cancer Immunotherapy. Small. 2020;16:2004879. doi: 10.1002/smll.202004879. [DOI] [PubMed] [Google Scholar]
- 209.Yip B. Recent Advances in CRISPR/Cas9 Delivery Strategies. Biomolecules. 2020;10:839. doi: 10.3390/biom10060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Conry R.M., LoBuglio A.F., Wright M., Sumerel L., Pike M.J., Johanning F., Benjamin R., Lu D., Curiel D.T. Characterization of a Messenger RNA Polynucleotide Vaccine Vector. Cancer Res. 1995;55:1397–1400. [PubMed] [Google Scholar]
- 211.Qin S., Tang X., Chen Y., Chen K., Fan N., Xiao W., Zheng Q., Li G., Teng Y., Wu M., et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. _target. Ther. 2022;7:166. doi: 10.1038/s41392-022-01007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Youn H., Chung J.-K. Modified mRNA as an alternative to plasmid DNA (pDNA) for transcript replacement and vaccination therapy. Expert Opin. Biol. Ther. 2015;15:1337–1348. doi: 10.1517/14712598.2015.1057563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Whitley J., Zwolinski C., Denis C., Maughan M., Hayles L., Clarke D., Snare M., Liao H., Chiou S., Marmura T., et al. Development of mRNA manufacturing for vaccines and therapeutics: mRNA platform requirements and development of a scalable production process to support early phase clinical trials. Transl. Res. 2022;242:38–55. doi: 10.1016/j.trsl.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kong N., Tao W., Ling X., Wang J., Xiao Y., Shi S., Ji X., Shajii A., Gan S.T., Kim N.Y., et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 2019;11:eaaw1565. doi: 10.1126/scitranslmed.aaw1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lin Y.-X., Wang Y., Ding J., Jiang A., Wang J., Yu M., Blake S., Liu S., Bieberich C.J., Farokhzad O.C., et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl. Med. 2021;13:eaba9772. doi: 10.1126/scitranslmed.aba9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Qiu M., Tang Y., Chen J., Muriph R., Ye Z., Huang C., Evans J., Henske E.P., Xu Q. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA. 2022;119:e2116271119. doi: 10.1073/pnas.2116271119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hu M., Feng C., Yuan Q., Liu C., Ge B., Sun F., Zhu X. Lantern-shaped flexible RNA origami for Smad4 mRNA delivery and growth suppression of colorectal cancer. Nat. Commun. 2023;14:1307. doi: 10.1038/s41467-023-37020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Van Hoecke L., Van Lint S., Roose K., Van Parys A., Vandenabeele P., Grooten J., Tavernier J., De Koker S., Saelens X. Treatment with mRNA coding for the necroptosis mediator MLKL induces antitumor immunity directed against neo-epitopes. Nat. Commun. 2018;9:3417. doi: 10.1038/s41467-018-05979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rybakova Y., Kowalski P.S., Huang Y., Gonzalez J.T., Heartlein M.W., DeRosa F., Delcassian D., Anderson D.G. mRNA Delivery for Therapeutic Anti-HER2 Antibody Expression In Vivo. Mol. Ther. 2019;27:1415–1423. doi: 10.1016/j.ymthe.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sayour E.J., Boczkowski D., Mitchell D.A., Nair S.K. Cancer mRNA vaccines: Clinical advances and future opportunities. Nat. Rev. Clin. Oncol. 2024;21:489–500. doi: 10.1038/s41571-024-00902-1. [DOI] [PubMed] [Google Scholar]
- 221.Liu J.-Q., Zhang C., Zhang X., Yan J., Zeng C., Talebian F., Lynch K., Zhao W., Hou X., Du S., et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control. Release. 2022;345:306–313. doi: 10.1016/j.jconrel.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hewitt S.L., Bai A., Bailey D., Ichikawa K., Zielinski J., Karp R., Apte A., Arnold K., Zacharek S.J., Iliou M.S., et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci. Transl. Med. 2019;11:eaat9143. doi: 10.1126/scitranslmed.aat9143. [DOI] [PubMed] [Google Scholar]
- 223.Hochmann S., Mittermeir M., Santic R., Koszik F., Griessner L., Sonderegger A.S., Hoffmann T., Russe E., Scheiblhofer S., Weiss R., et al. Evaluation of modified Interferon alpha mRNA constructs for the treatment of non-melanoma skin cancer. Sci. Rep. 2018;8:12954. doi: 10.1038/s41598-018-31061-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fan Y.-N., Li M., Luo Y.-L., Chen Q., Wang L., Zhang H.-B., Shen S., Gu Z., Wang J. Cationic lipid-assisted nanoparticles for delivery of mRNA cancer vaccine. Biomater. Sci. 2018;6:3009–3018. doi: 10.1039/C8BM00908B. [DOI] [PubMed] [Google Scholar]
- 225.Mai Y., Guo J., Zhao Y., Ma S., Hou Y., Yang J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell. Immunol. 2020;354:104143. doi: 10.1016/j.cellimm.2020.104143. [DOI] [PubMed] [Google Scholar]
- 226.Liu L., Wang Y., Miao L., Liu Q., Musetti S., Li J., Huang L. Combination Immunotherapy of MUC1 mRNA Nano-vaccine and CTLA-4 Blockade Effectively Inhibits Growth of Triple Negative Breast Cancer. Mol. Ther. 2018;26:45–55. doi: 10.1016/j.ymthe.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wu L., Wang W., Tian J., Qi C., Cai Z., Yan W., Xuan S., Shang A. Engineered mRNA-expressed bispecific antibody prevent intestinal cancer via lipid nanoparticle delivery. Bioengineered. 2021;12:12383–12393. doi: 10.1080/21655979.2021.2003666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang Y., Peng Y., Zi G., Chen J., Peng B. Co-delivery of Cas9 mRNA and guide RNAs for editing of LGMN gene represses breast cancer cell metastasis. Sci. Rep. 2024;14:8095. doi: 10.1038/s41598-024-58765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Reyes-Darias J.A., Berzal-Herranz A. Detection of immune response activation by exogenous nucleic acids by a multiplex RT-PCR method. Mol. Cell. Probes. 2014;28:181–185. doi: 10.1016/j.mcp.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 230.Kay M.A., Glorioso J.C., Naldini L. Viral vectors for gene therapy: The art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 231.Ghosh S., Brown A.M., Jenkins C., Campbell K. Viral Vector Systems for Gene Therapy: A Comprehensive Literature Review of Progress and Biosafety Challenges. Appl. Biosaf. 2020;25:7–18. doi: 10.1177/1535676019899502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lee C.S., Bishop E.S., Zhang R., Yu X., Farina E.M., Yan S., Zhao C., Zeng Z., Shu Y., Wu X., et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4:43–63. doi: 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bulcha J.T., Wang Y., Ma H., Tai P.W.L., Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct. _target. Ther. 2021;6:53. doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lv Z., Zhu Y., Li F. DNA Functional Nanomaterials for Controlled Delivery of Nucleic Acid-Based Drugs. Front. Bioeng. Biotechnol. 2021;9:720291. doi: 10.3389/fbioe.2021.720291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li C., Luo S., Wang J., Shen Z., Wu Z.-S. Nuclease-resistant signaling nanostructures made entirely of DNA oligonucleotides. Nanoscale. 2021;13:7034–7051. doi: 10.1039/D1NR00197C. [DOI] [PubMed] [Google Scholar]
- 236.Morya V., Walia S., Mandal B.B., Ghoroi C., Bhatia D. Functional DNA Based Hydrogels: Development, Properties and Biological Applications. ACS Biomater. Sci. Eng. 2020;6:6021–6035. doi: 10.1021/acsbiomaterials.0c01125. [DOI] [PubMed] [Google Scholar]
- 237.Fu X., Chen T., Song Y., Feng C., Chen H., Zhang Q., Chen G., Zhu X. mRNA Delivery by a pH-Responsive DNA Nano-Hydrogel. Small. 2021;17:2101224. doi: 10.1002/smll.202101224. [DOI] [PubMed] [Google Scholar]
- 238.Song J., Lee M., Kim T., Na J., Jung Y., Jung G.Y., Kim S., Park N. A RNA producing DNA hydrogel as a platform for a high performance RNA interference system. Nat. Commun. 2018;9:4331. doi: 10.1038/s41467-018-06864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Choi C.H.J., Hao L., Narayan S.P., Auyeung E., Mirkin C.A. Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proc. Natl. Acad. Sci. USA. 2013;110:7625–7630. doi: 10.1073/pnas.1305804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Brodin J.D., Sprangers A.J., McMillan J.R., Mirkin C.A. DNA-Mediated Cellular Delivery of Functional Enzymes. J. Am. Chem. Soc. 2015;137:14838–14841. doi: 10.1021/jacs.5b09711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang T., Liu Y., Wu Q., Lou B., Liu Z. DNA nanostructures for stimuli-responsive drug delivery. Smart Mater. Med. 2021;3:66–84. doi: 10.1016/j.smaim.2021.12.003. [DOI] [Google Scholar]
- 242.Han X., Xu X., Wu Z., Wu Z., Qi X. Synchronous conjugation of i-motif DNA and therapeutic siRNA on the vertexes of tetrahedral DNA nanocages for efficient gene silence. Acta Pharm. Sin. B. 2021;11:3286–3296. doi: 10.1016/j.apsb.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jiang Y., Huo S., Hardie J., Liang X.-J., Rotello V.M. Progress and perspective of inorganic nanoparticle-based siRNA delivery systems. Expert Opin. Drug Deliv. 2016;13:547–559. doi: 10.1517/17425247.2016.1134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jiang Z., Thayumanavan S. Noncationic Material Design for Nucleic Acid Delivery. Adv. Ther. 2020;3:1900206. doi: 10.1002/adtp.201900206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ding Y., Jiang Z., Saha K., Kim C.S., Kim S.T., Landis R.F., Rotello V.M. Gold Nanoparticles for Nucleic Acid Delivery. Mol. Ther. 2014;22:1075–1083. doi: 10.1038/mt.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ma X., Li X., Luo G., Jiao J. DNA-functionalized gold nanoparticles: Modification, characterization, and biomedical applications. Front. Chem. 2022;10:1095488. doi: 10.3389/fchem.2022.1095488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Amendola V., Pilot R., Frasconi M., Maragò O.M., Iatì M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter. 2017;29:203002. doi: 10.1088/1361-648X/aa60f3. [DOI] [PubMed] [Google Scholar]
- 248.Kus-Liśkiewicz M., Fickers P., Tahar I.B. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021;22:10952. doi: 10.3390/ijms222010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Luther D.C., Huang R., Jeon T., Zhang X., Lee Y.-W., Nagaraj H., Rotello V.M. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020;156:188–213. doi: 10.1016/j.addr.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Torres-Vanegas J.D., Cruz J.C., Reyes L.H. Delivery Systems for Nucleic Acids and Proteins: Barriers, Cell Capture Pathways and Nanocarriers. Pharmaceutics. 2021;13:428. doi: 10.3390/pharmaceutics13030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dong S., Feng Z., Ma R., Zhang T., Jiang J., Li Y., Zhang Y., Li S., Liu X., Liu X., et al. Engineered Design of a Mesoporous Silica Nanoparticle-Based Nanocarrier for Efficient mRNA Delivery in Vivo. Nano Lett. 2023;23:2137–2147. doi: 10.1021/acs.nanolett.2c04486. [DOI] [PubMed] [Google Scholar]
- 252.Zhang J., Lei W., Meng Y., Zhou C., Zhang B., Yuan J., Wang M., Xu D., Meng X., Chen W. Expression of PEI-coated gold nanoparticles carrying exogenous gene in periwinkle mesophyll cells and its practice in huanglongbing research. iScience. 2022;25:104479. doi: 10.1016/j.isci.2022.104479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Aschmann D., Knol R.A., Kros A. Lipid-Based Nanoparticle Functionalization with Coiled-Coil Peptides for In Vitro and In Vivo Drug Delivery. Acc. Chem. Res. 2024;57:1098–1110. doi: 10.1021/acs.accounts.3c00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kiaie S.H., Zolbanin N.M., Ahmadi A., Bagherifar R., Valizadeh H., Kashanchi F., Jafari R. Recent advances in mRNA-LNP therapeutics: Immunological and pharmacological aspects. J. Nanobiotechnology. 2022;20:276. doi: 10.1186/s12951-022-01478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yonezawa S., Koide H., Asai T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020;154–155:64–78. doi: 10.1016/j.addr.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Samaridou E., Heyes J., Lutwyche P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020;154–155:37–63. doi: 10.1016/j.addr.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 257.Albertsen C.H., Kulkarni J.A., Witzigmann D., Lind M., Petersson K., Simonsen J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022;188:114416. doi: 10.1016/j.addr.2022.114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Parhiz H., Shuvaev V.V., Pardi N., Khoshnejad M., Kiseleva R.Y., Brenner J.S., Uhler T., Tuyishime S., Mui B.L., Tam Y.K., et al. PECAM-1 directed re-_targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J. Control. Release. 2018;291:106–115. doi: 10.1016/j.jconrel.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nsairat H., Mahmoud I.S., Odeh F., Abuarqoub D., Al-Azzawi H., Zaza R., Qadri M.I., Ismail S., Bawab A.A., Awidi A., et al. Grafting of anti-nucleolin aptamer into preformed and remotely loaded liposomes through aptamer-cholesterol post-insertion. RSC Adv. 2020;10:36219–36229. doi: 10.1039/D0RA07325C. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study.