The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region
- PMID: 10468583
- PMCID: PMC17863
- DOI: 10.1073/pnas.96.18.10182
The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region
Abstract
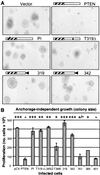
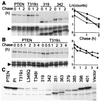
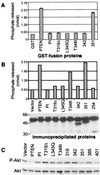
PTEN is a recently identified tumor suppressor inactivated in a variety of cancers such as glioblastoma and endometrial and prostate carcinoma. It contains an amino-terminal phosphatase domain and acts as a phosphatidylinositol 3,4,5-trisphosphate phosphatase antagonizing the activity of the phosphatidylinositol 3-OH kinase. PTEN also contains a carboxyl-terminal domain, and we addressed the role of this region that, analogous to the amino-terminal phosphatase domain, is the _target of many mutations identified in tumors. Expression of carboxyl-terminal mutants in PTEN-deficient glioblastoma cells permitted the anchorage-independent growth of the cells that otherwise was suppressed by wild-type PTEN. The stability of these mutants in cells was reduced because of rapid degradation. Although the carboxyl-terminal region contains regulatory PEST sequences and a PDZ-binding motif, these specific elements were dispensable for the tumor-suppressor function. The study of carboxyl-terminal point mutations affecting the stability of PTEN revealed that these were located in strongly predicted beta-strands. Surprisingly, the phosphatase activity of these mutants was affected in correlation with the degree of disruption of these structural elements. We conclude that the carboxyl-terminal region is essential for regulating PTEN stability and enzymatic activity and that mutations in this region are responsible for the reversion of the tumor-suppressor phenotype. We also propose that the molecular conformational changes induced by these mutations constitute the mechanism for PTEN inactivation.
Figures


Similar articles
-
Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor.Cancer Res. 2000 Dec 15;60(24):7033-8. Cancer Res. 2000. PMID: 11156408
-
_targeting mutants of PTEN reveal distinct subsets of tumour suppressor functions.Biochem J. 2001 Jul 15;357(Pt 2):427-35. doi: 10.1042/0264-6021:3570427. Biochem J. 2001. PMID: 11439092 Free PMC article.
-
PTEN 2, a Golgi-associated testis-specific homologue of the PTEN tumor suppressor lipid phosphatase.J Biol Chem. 2001 Jun 15;276(24):21745-53. doi: 10.1074/jbc.M101480200. Epub 2001 Mar 2. J Biol Chem. 2001. PMID: 11279206
-
The tumour suppressor PTEN: involvement of a tumour suppressor candidate protein in PTEN turnover.Biochem Soc Trans. 2004 Apr;32(Pt 2):343-7. doi: 10.1042/bst0320343. Biochem Soc Trans. 2004. PMID: 15046605 Review.
-
PTEN: a novel anti-oncogenic function independent of phosphatase activity.Cell Cycle. 2005 Apr;4(4):540-2. doi: 10.4161/cc.4.4.1614. Epub 2005 Apr 21. Cell Cycle. 2005. PMID: 15753657 Review.
Cited by
-
TPIP: a novel phosphoinositide 3-phosphatase.Biochem J. 2001 Dec 1;360(Pt 2):277-83. doi: 10.1042/0264-6021:3600277. Biochem J. 2001. PMID: 11716755 Free PMC article.
-
Oxidative stress-induced expression and modulation of Phosphatase of Regenerating Liver-1 (PRL-1) in mammalian retina.Biochim Biophys Acta. 2007 Sep;1773(9):1473-82. doi: 10.1016/j.bbamcr.2007.06.005. Epub 2007 Jun 26. Biochim Biophys Acta. 2007. PMID: 17673310 Free PMC article.
-
Three-dimensional tissue culture based on magnetic cell levitation.Nat Nanotechnol. 2010 Apr;5(4):291-6. doi: 10.1038/nnano.2010.23. Epub 2010 Mar 14. Nat Nanotechnol. 2010. PMID: 20228788 Free PMC article.
-
Akt versus p53 in a network of oncogenes and tumor suppressor genes regulating cell survival and death.Biophys J. 2006 Aug 1;91(3):857-65. doi: 10.1529/biophysj.105.077693. Epub 2006 Apr 28. Biophys J. 2006. PMID: 16648169 Free PMC article.
-
Protean PTEN: form and function.Am J Hum Genet. 2002 Apr;70(4):829-44. doi: 10.1086/340026. Epub 2002 Mar 1. Am J Hum Genet. 2002. PMID: 11875759 Free PMC article. Review.
References
-
- Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. - PubMed
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. - PubMed
-
- Rasheed B K, Stenzel T T, McLendon R E, Parsons R, Friedman A H, Friedman H S, Bigner D D, Bigner S H. Cancer Res. 1997;57:4187–4190. - PubMed
-
- Wang S I, Puc J, Li J, Bruce J N, Cairns P, Sidransky D, Parsons R. Cancer Res. 1997;57:4183–4186. - PubMed
-
- Tashiro H, Blazes M S, Wu R, Cho K R, Bose S, Wang S I, Li J, Parsons R, Ellenson L H. Cancer Res. 1997;57:3935–3940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

