Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing
- PMID: 10632593
- PMCID: PMC6772395
- DOI: 10.1523/JNEUROSCI.20-02-00639.2000
Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing
Abstract
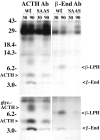
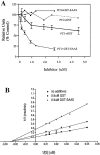
Five novel peptides were identified in the brains of mice lacking active carboxypeptidase E, a neuropeptide-processing enzyme. These peptides are produced from a single precursor, termed proSAAS, which is present in human, mouse, and rat. ProSAAS mRNA is expressed primarily in brain and other neuroendocrine tissues (pituitary, adrenal, pancreas); within brain, the mRNA is broadly distributed among neurons. When expressed in AtT-20 cells, proSAAS is secreted via the regulated pathway and is also processed at paired-basic cleavage sites into smaller peptides. Overexpression of proSAAS in the AtT-20 cells substantially reduces the rate of processing of the endogenous prohormone proopiomelanocortin. Purified proSAAS inhibits prohormone convertase 1 activity with an IC(50) of 590 nM but does not inhibit prohormone convertase 2. Taken together, proSAAS may represent an endogenous inhibitor of prohormone convertase 1.
Figures

Similar articles
-
Distribution of proSAAS-derived peptides in rat neuroendocrine tissues.Neuroscience. 2001;105(2):469-78. doi: 10.1016/s0306-4522(01)00200-7. Neuroscience. 2001. PMID: 11672612
-
Processing of proSAAS in neuroendocrine cell lines.Biochem J. 2002 Jan 1;361(Pt 1):67-76. doi: 10.1042/0264-6021:3610067. Biochem J. 2002. PMID: 11742530 Free PMC article.
-
Tissue distribution and processing of proSAAS by proprotein convertases.J Neurochem. 2001 Mar;76(6):1833-41. doi: 10.1046/j.1471-4159.2001.00165.x. J Neurochem. 2001. PMID: 11259501
-
Post-translational processing of proopiomelanocortin in the pituitary and in the brain.Crit Rev Neurobiol. 1997;11(1):35-57. doi: 10.1615/critrevneurobiol.v11.i1.30. Crit Rev Neurobiol. 1997. PMID: 9093813 Review.
-
On the discovery of precursor processing.Methods Mol Biol. 2011;768:3-11. doi: 10.1007/978-1-61779-204-5_1. Methods Mol Biol. 2011. PMID: 21805235 Review.
Cited by
-
Discovery of Human Signaling Systems: Pairing Peptides to G Protein-Coupled Receptors.Cell. 2019 Oct 31;179(4):895-908.e21. doi: 10.1016/j.cell.2019.10.010. Cell. 2019. PMID: 31675498 Free PMC article.
-
Strategies for the Identification of Bioactive Neuropeptides in Vertebrates.Front Neurosci. 2019 Sep 18;13:948. doi: 10.3389/fnins.2019.00948. eCollection 2019. Front Neurosci. 2019. PMID: 31619945 Free PMC article. Review.
-
The extended granin family: structure, function, and biomedical implications.Endocr Rev. 2011 Dec;32(6):755-97. doi: 10.1210/er.2010-0027. Epub 2011 Aug 23. Endocr Rev. 2011. PMID: 21862681 Free PMC article. Review.
-
A reduced proteomic signature in critically ill Covid-19 patients determined with plasma antibody micro-array and machine learning.Clin Proteomics. 2024 May 17;21(1):33. doi: 10.1186/s12014-024-09488-3. Clin Proteomics. 2024. PMID: 38760690 Free PMC article.
-
60 YEARS OF POMC: Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides.J Mol Endocrinol. 2016 May;56(4):T77-97. doi: 10.1530/JME-15-0323. Epub 2016 Feb 15. J Mol Endocrinol. 2016. PMID: 26880796 Free PMC article. Review.
References
-
- Bauerfeind R, Huttner WB. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993;5:628–635. - PubMed
-
- Benjannet S, Savaria D, Chretien M, Seidah NG. 7B2 is a specific intracellular binding protein of the prohormone convertase PC2. J Neurochem. 1995;64:2303–2311. - PubMed
-
- Bennett HPJ. Glycosylation, phosphorylation, and sulfation of peptide hormones and their precursors. In: Fricker LD, editor. Peptide biosynthesis and processing. CRC; Boca Raton: 1991. pp. 111–140.
-
- Boudreault A, Gauthier D, Lazure C. Proprotein convertase PC1/3-related peptides are potent slow tight-binding inhibitors of murine PC1/3 and Hfurin. J Biol Chem. 1998a;273:31574–31580. - PubMed
-
- Boudreault A, Gauthier D, Rondeau N, Savaria D, Seidah NG, Chretien M, Lazure C. Molecular characterization, enzymatic analysis, and purification of murine proprotein convertase-1/3 (PC1/3) secreted from recombinant baculovirus-infected insect cells. Protein Expr Purif. 1998b;14:353–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
