Herpes simplex virus ICP27 induces cytoplasmic accumulation of unspliced polyadenylated alpha-globin pre-mRNA in infected HeLa cells
- PMID: 10684311
- PMCID: PMC111785
- DOI: 10.1128/jvi.74.6.2913-2919.2000
Herpes simplex virus ICP27 induces cytoplasmic accumulation of unspliced polyadenylated alpha-globin pre-mRNA in infected HeLa cells
Abstract
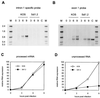
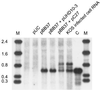
Transcripts of most intron-bearing cellular genes must be processed by the splicing machinery in order to efficiently accumulate and gain access to the cytoplasm. However, we found that herpes simplex virus induces cytoplasmic accumulation of both spliced and unspliced polyadenylated alpha-globin RNAs in infected HeLa cells. Accumulation of the unspliced RNA required the immediate-early protein ICP27, and ICP27 was sufficient (in combination with ICP4) to produce this effect in a transient-transfection assay. However, expression of ICP27 did not markedly alter the levels of fully spliced alpha-globin transcripts in infected cells. These data demonstrate that the previously documented effects of ICP27 on the cellular splicing apparatus do not greatly inhibit splicing of alpha-globin RNA and argue that ICP27 induces a splicing-independent pathway for alpha-globin RNA accumulation and nuclear export.
Figures
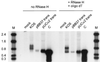


Similar articles
-
Processing of alpha-globin and ICP0 mRNA in cells infected with herpes simplex virus type 1 ICP27 mutants.J Virol. 2000 Aug;74(16):7307-19. doi: 10.1128/jvi.74.16.7307-7319.2000. J Virol. 2000. PMID: 10906184 Free PMC article.
-
Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect.J Virol. 1994 Dec;68(12):7790-9. doi: 10.1128/JVI.68.12.7790-7799.1994. J Virol. 1994. PMID: 7966568 Free PMC article.
-
ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif.Genes Dev. 1998 Mar 15;12(6):868-79. doi: 10.1101/gad.12.6.868. Genes Dev. 1998. PMID: 9512520 Free PMC article.
-
The many roles of the regulatory protein ICP27 during herpes simplex virus infection.Front Biosci. 2008 May 1;13:5241-56. doi: 10.2741/3078. Front Biosci. 2008. PMID: 18508584 Review.
-
Properties of an HSV-1 regulatory protein that appears to impair host cell splicing.Infect Agents Dis. 1994 Apr-Jun;3(2-3):59-67. Infect Agents Dis. 1994. PMID: 7812656 Review.
Cited by
-
A Review of the Multipronged Attack of Herpes Simplex Virus 1 on the Host Transcriptional Machinery.Viruses. 2021 Sep 14;13(9):1836. doi: 10.3390/v13091836. Viruses. 2021. PMID: 34578417 Free PMC article. Review.
-
Mapping of functional regions in the amino-terminal portion of the herpes simplex virus ICP27 regulatory protein: importance of the leucine-rich nuclear export signal and RGG Box RNA-binding domain.J Virol. 2002 Dec;76(23):11866-79. doi: 10.1128/jvi.76.23.11866-11879.2002. J Virol. 2002. PMID: 12414929 Free PMC article.
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
-
Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells.J Virol. 2001 Jan;75(2):1013-30. doi: 10.1128/JVI.75.2.1013-1030.2001. J Virol. 2001. PMID: 11134315 Free PMC article.
-
Processing of alpha-globin and ICP0 mRNA in cells infected with herpes simplex virus type 1 ICP27 mutants.J Virol. 2000 Aug;74(16):7307-19. doi: 10.1128/jvi.74.16.7307-7319.2000. J Virol. 2000. PMID: 10906184 Free PMC article.
References
-
- Burgess S M, Guthrie C. A mechanism to enhance mRNA splicing fidelity: the RNA-dependent ATPase Prp16 governs usage of a discard pathway for aberrant lariat intermediates. Cell. 1993;73:1377–1391. - PubMed
-
- Chang D D, Sharp P A. Regulation by HIV Rev depends upon recognition of splice sites. Cell. 1989;59:789–795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

