Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype
- PMID: 10861013
- PMCID: PMC16568
- DOI: 10.1073/pnas.97.13.7464
Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype
Abstract
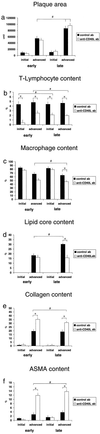
In the present study, we investigated the role of the CD40L-CD40 pathway in a model of progressive atherosclerosis. ApoE-/- mice were treated with an anti-CD40L antibody or a control antibody for 12 wk. Antibody treatment started early (age 5 wk) or was delayed until after the establishment of atherosclerosis (age 17 wk). In both the early and delayed treatment groups, anti-CD40L antibody did not decrease plaque area or inhibit lesion initiation or age-related increase in lesion area. The morphology of initial lesions was not affected, except for a decrease in T-lymphocyte content. Effects of anti-CD40L antibody treatment on the morphology of advanced lesions were pronounced. In both the early and delayed treatment groups, T-lymphocyte content was significantly decreased. Furthermore, a pronounced increase in collagen content, vascular smooth muscle cell/myofibroblast content, and fibrous cap thickness was observed. In the delayed treatment group, a decrease in lipid core and macrophage content occurred. Interestingly, advanced lesions of anti-CD40L antibody-treated mice exhibited an increased transforming growth factor beta1 immunoreactivity, especially in macrophages. In conclusion, both early and delayed treatment with an anti-CD40L antibody do not affect atherosclerotic lesion initiation but do result in the development of a lipid-poor collagen-rich stable plaque phenotype. Furthermore, delayed treatment with anti-CD40L antibody can transform the lesion profile from a lipid-rich to a lipid-poor collagen-rich phenotype. Postulated mechanisms of this effect on plaque phenotype are the down-regulation of proinflammatory pathways and up-regulation of collagen-promoting factors like transforming growth factor beta.
Figures

Comment in
-
Atherosclerosis: the emerging role of inflammation and the CD40-CD40 ligand system.Proc Natl Acad Sci U S A. 2000 Jun 20;97(13):6930-2. doi: 10.1073/pnas.97.13.6930. Proc Natl Acad Sci U S A. 2000. PMID: 10860949 Free PMC article. No abstract available.
Similar articles
-
Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice.Proc Natl Acad Sci U S A. 2000 Jun 20;97(13):7458-63. doi: 10.1073/pnas.97.13.7458. Proc Natl Acad Sci U S A. 2000. PMID: 10861012 Free PMC article.
-
Reduction of atherosclerosis in mice by inhibition of CD40 signalling.Nature. 1998 Jul 9;394(6689):200-3. doi: 10.1038/28204. Nature. 1998. PMID: 9671306
-
CD40-CD40L interactions in atherosclerosis.Trends Cardiovasc Med. 2002 Jan;12(1):27-32. doi: 10.1016/s1050-1738(01)00142-6. Trends Cardiovasc Med. 2002. PMID: 11796241 Review.
-
Rosuvastatin stabilizes atherosclerotic plaques by reducing CD40L overexpression-induced downregulation of P4Hα1 in ApoE-/- mice.Int J Biochem Cell Biol. 2018 Dec;105:70-77. doi: 10.1016/j.biocel.2018.10.002. Epub 2018 Oct 15. Int J Biochem Cell Biol. 2018. PMID: 30336263
-
CD40 signaling in vascular cells: a key role in atherosclerosis?Atherosclerosis. 1998 Apr;137 Suppl:S89-95. doi: 10.1016/s0021-9150(97)00309-2. Atherosclerosis. 1998. PMID: 9694547 Review.
Cited by
-
Abrogated transforming growth factor beta receptor II (TGFβRII) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis.Eur Heart J. 2013 Dec;34(48):3717-27. doi: 10.1093/eurheartj/ehs106. Epub 2012 May 21. Eur Heart J. 2013. PMID: 22613345 Free PMC article.
-
Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling.Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12367-71. doi: 10.1073/pnas.2032886100. Epub 2003 Sep 30. Proc Natl Acad Sci U S A. 2003. PMID: 14519852 Free PMC article.
-
Interleukin-1 Beta as a _target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond.J Am Coll Cardiol. 2017 Oct 31;70(18):2278-2289. doi: 10.1016/j.jacc.2017.09.028. J Am Coll Cardiol. 2017. PMID: 29073957 Free PMC article. Review.
-
CD40L and Its Receptors in Atherothrombosis-An Update.Front Cardiovasc Med. 2017 Jun 20;4:40. doi: 10.3389/fcvm.2017.00040. eCollection 2017. Front Cardiovasc Med. 2017. PMID: 28676852 Free PMC article. Review.
-
The role of platelets in the recruitment of leukocytes during vascular disease.Platelets. 2015;26(6):507-20. doi: 10.3109/09537104.2015.1064881. Epub 2015 Jul 21. Platelets. 2015. PMID: 26196409 Free PMC article. Review.
References
-
- Foy T M, Aruffo A, Bajorath J, Buhlmann J E, Noelle R J. Annu Rev Immunol. 1996;14:591–617. - PubMed
-
- Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, Bajorath J, Grosmaire L S, Stenkamp R, Neubauer M, et al. Cell. 1993;72:291–300. - PubMed
-
- Durie F H, Fava R A, Foy T M, Aruffo A, Ledbetter J A, Noelle R J. Science. 1993;261:1328–1330. - PubMed
-
- Larsen C P, Elwood E T, Alexander D Z, Ritchie S C, Hendrix R, Tucker-Burder C, Cho H R, Aruffo A, Hollenbaugh D, Linsley P S, et al. Nature (London) 1996;381:434–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

