Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha -synuclein in human and transgenic mouse brain
- PMID: 10964942
- PMCID: PMC6772969
- DOI: 10.1523/JNEUROSCI.20-17-06365.2000
Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha -synuclein in human and transgenic mouse brain
Abstract
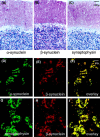
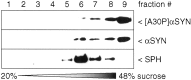
Mutations in the alpha-synuclein (alphaSYN) gene are associated with rare cases of familial Parkinson's disease, and alphaSYN is a major component of Lewy bodies and Lewy neurites. Here we have investigated the localization of wild-type and mutant [A30P]alphaSYN as well as betaSYN at the cellular and subcellular level. Our direct comparative study demonstrates extensive synaptic colocalization of alphaSYN and betaSYN in human and mouse brain. In a sucrose gradient equilibrium centrifugation assay, a portion of betaSYN floated into lower density fractions, which also contained the synaptic vesicle marker synaptophysin. Likewise, wild-type and [A30P]alphaSYN were found in floating fractions. Subcellular fractionation of mouse brain revealed that both alphaSYN and betaSYN were present in synaptosomes. In contrast to synaptophysin, betaSYN and alphaSYN were recovered from the soluble fraction upon lysis of the synaptosomes. Synaptic colocalization of alphaSYN and betaSYN was directly visualized by confocal microscopy of double-stained human brain sections. The Parkinson's disease-associated human mutant [A30P]alphaSYN was found to colocalize with betaSYN and synaptophysin in synapses of transgenic mouse brain. However, in addition to their normal presynaptic localization, transgenic wild-type and [A30P]alphaSYN abnormally accumulated in neuronal cell bodies and neurites throughout the brain. Thus, mutant [A30P]alphaSYN does not fail to be transported to synapses, but its transgenic overexpression apparently leads to abnormal cellular accumulations.
Figures





Similar articles
-
Sensitivity to MPTP is not increased in Parkinson's disease-associated mutant alpha-synuclein transgenic mice.J Neurochem. 2001 May;77(4):1181-4. doi: 10.1046/j.1471-4159.2001.00366.x. J Neurochem. 2001. PMID: 11359883
-
Effect of familial Parkinson's disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein.Biochemistry. 2001 Sep 25;40(38):11604-13. doi: 10.1021/bi010616g. Biochemistry. 2001. PMID: 11560511
-
Nuclear and neuritic distribution of serine-129 phosphorylated alpha-synuclein in transgenic mice.Neuroscience. 2009 Jun 2;160(4):796-804. doi: 10.1016/j.neuroscience.2009.03.002. Epub 2009 Mar 9. Neuroscience. 2009. PMID: 19272424
-
Physiology and pathophysiology of alpha-synuclein. Cell culture and transgenic animal models based on a Parkinson's disease-associated protein.Ann N Y Acad Sci. 2000;920:33-41. doi: 10.1111/j.1749-6632.2000.tb06902.x. Ann N Y Acad Sci. 2000. PMID: 11193173 Review.
-
Properties of NACP/alpha-synuclein and its role in Alzheimer's disease.Biochim Biophys Acta. 2000 Jul 26;1502(1):95-109. doi: 10.1016/s0925-4439(00)00036-3. Biochim Biophys Acta. 2000. PMID: 10899435 Review.
Cited by
-
The prion hypothesis of Parkinson's disease.Curr Neurol Neurosci Rep. 2015 May;15(5):28. doi: 10.1007/s11910-015-0549-x. Curr Neurol Neurosci Rep. 2015. PMID: 25868519
-
KTKEGV repeat motifs are key mediators of normal α-synuclein tetramerization: Their mutation causes excess monomers and neurotoxicity.Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):9596-601. doi: 10.1073/pnas.1505953112. Epub 2015 Jul 7. Proc Natl Acad Sci U S A. 2015. PMID: 26153422 Free PMC article.
-
SUMOylation in α-Synuclein Homeostasis and Pathology.Front Aging Neurosci. 2020 Jun 25;12:167. doi: 10.3389/fnagi.2020.00167. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32670048 Free PMC article. Review.
-
Genetics of parkinsonism.Curr Neurol Neurosci Rep. 2005 Sep;5(5):397-404. doi: 10.1007/s11910-005-0064-6. Curr Neurol Neurosci Rep. 2005. PMID: 16131423 Review.
-
Acyl-CoA synthetase activity links wild-type but not mutant alpha-synuclein to brain arachidonate metabolism.Biochemistry. 2006 Jun 6;45(22):6956-66. doi: 10.1021/bi0600289. Biochemistry. 2006. PMID: 16734431 Free PMC article.
References
-
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho W-H, Castillo PE, Shinsky N, Garcia Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Arawaka S, Saito Y, Murayama S, Mori H. Lewy body in neurodegeneration with brain iron accumulation type 1 is immunoreactive for α-synuclein. Neurology. 1998;51:887–889. - PubMed
-
- Arima K, Uéda K, Sunohara N, Hirai S, Izumiyama Y, Tonozuka-Uehara H, Kawai M. Immunoelectron-microscopic demonstration of NACP/α-synuclein-epitopes on the filamentous component of Lewy bodies in Parkinson's disease and in dementia with Lewy bodies. Brain Res. 1998;808:93–100. - PubMed
-
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
