Inhibition of Daxx-mediated apoptosis by heat shock protein 27
- PMID: 11003656
- PMCID: PMC86317
- DOI: 10.1128/MCB.20.20.7602-7612.2000
Inhibition of Daxx-mediated apoptosis by heat shock protein 27
Abstract
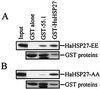
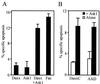
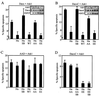
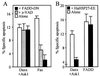
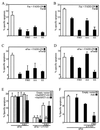
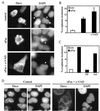
Heat shock protein 27 (HSP27) confers cellular protection against a variety of cytotoxic stresses and also against physiological stresses associated with growth arrest or receptor-mediated apoptosis. Phosphorylation modulates the activity of HSP27 by causing a major change in the supramolecular organization of the protein, which shifts from oligomers to dimers. Here we show that phosphorylated dimers of HSP27 interact with Daxx, a mediator of Fas-induced apoptosis, preventing the interaction of Daxx with both Ask1 and Fas and blocking Daxx-mediated apoptosis. No such inhibition was observed with an HSP27 phosphorylation mutant that is only expressed as oligomers or when apoptosis was induced by transfection of a Daxx mutant lacking its HSP27 binding domain. HSP27 expression had no effect on Fas-induced FADD- and caspase-dependent apoptosis. However, HSP27 blocked Fas-induced translocation of Daxx from the nucleus to the cytoplasm and Fas-induced Daxx- and Ask1-dependent apoptosis. The observations revealed a new level of regulation of the Fas pathway and suggest a mechanism for the phosphorylation-dependent protective function of HSP27 during stress and differentiation.
Figures

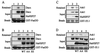
Similar articles
-
The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis.Ann N Y Acad Sci. 2000;926:126-31. doi: 10.1111/j.1749-6632.2000.tb05606.x. Ann N Y Acad Sci. 2000. PMID: 11193028
-
A kinase-independent function of Ask1 in caspase-independent cell death.J Biol Chem. 2001 Sep 28;276(39):36071-4. doi: 10.1074/jbc.C100340200. Epub 2001 Aug 7. J Biol Chem. 2001. PMID: 11493600
-
Apoptosis signal-regulating kinase 1 controls the proapoptotic function of death-associated protein (Daxx) in the cytoplasm.J Biol Chem. 2001 Oct 19;276(42):39103-6. doi: 10.1074/jbc.M105928200. Epub 2001 Aug 8. J Biol Chem. 2001. PMID: 11495919
-
Daxx: death or survival protein?Trends Cell Biol. 2006 Feb;16(2):97-104. doi: 10.1016/j.tcb.2005.12.002. Epub 2006 Jan 10. Trends Cell Biol. 2006. PMID: 16406523 Review.
-
The Daxx enigma.Apoptosis. 2000 Jun;5(3):217-20. doi: 10.1023/a:1009696227420. Apoptosis. 2000. PMID: 11225842 Review.
Cited by
-
Quantitative phosphoproteomic study of pressure-overloaded mouse heart reveals dynamin-related protein 1 as a modulator of cardiac hypertrophy.Mol Cell Proteomics. 2013 Nov;12(11):3094-107. doi: 10.1074/mcp.M113.027649. Epub 2013 Jul 23. Mol Cell Proteomics. 2013. PMID: 23882026 Free PMC article.
-
Network-based proteomic approaches reveal the neurodegenerative, neuroprotective and pain-related mechanisms involved after retrograde axonal damage.Sci Rep. 2015 Mar 18;5:9185. doi: 10.1038/srep09185. Sci Rep. 2015. PMID: 25784190 Free PMC article.
-
Reduced DAXX Expression Is Associated with Reduced CD24 Expression in Colorectal Cancer.Cells. 2019 Oct 12;8(10):1242. doi: 10.3390/cells8101242. Cells. 2019. PMID: 31614769 Free PMC article.
-
Serine 59 phosphorylation of {alpha}B-crystallin down-regulates its anti-apoptotic function by binding and sequestering Bcl-2 in breast cancer cells.J Biol Chem. 2010 Nov 26;285(48):37324-32. doi: 10.1074/jbc.M110.124388. Epub 2010 Sep 14. J Biol Chem. 2010. PMID: 20841355 Free PMC article.
-
The protective role of HSP27 in ocular diseases.Mol Biol Rep. 2022 Jun;49(6):5107-5115. doi: 10.1007/s11033-022-07222-6. Epub 2022 Feb 25. Mol Biol Rep. 2022. PMID: 35212927 Review.
References
-
- Algeciras-Schimnich A, Griffith T S, Lynch D H, Paya C V. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J Immunol. 1999;162:5205–5211. - PubMed
-
- Arrigo A P, Landry J. Expression and function of the low-molecular-weight heat shock proteins. In: Morimoto R I, Tissières A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 335–373.
-
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. - PubMed
-
- Benndorf R, Kraft R, Otto A, Stahl J, Bohm H, Bielka H. Purification of the growth-related protein p25 of the Ehrlich ascites tumor and analysis of its isoforms. Biochem Int. 1988;17:225–234. - PubMed
-
- Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1 and TNF receptor-induced cell death. Cell. 1996;85:803–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
