Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation
- PMID: 11141488
- PMCID: PMC1850249
- DOI: 10.1016/S0002-9440(10)63953-3
Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation
Abstract
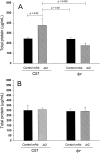
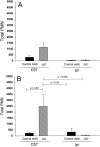
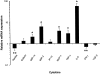
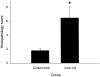
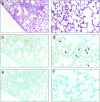
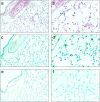
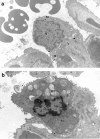
Activation of the Fas/FasL system induces apoptosis of susceptible cells, but may also lead to nuclear factor kappaB activation. Our goal was to determine whether local Fas activation produces acute lung injury by inducing alveolar epithelial cell apoptosis and by generating local inflammatory responses. Normal mice (C57BL/6) and mice deficient in Fas (lpr) were treated by intranasal instillation of the Fas-activating monoclonal antibody (mAb) Jo2 or an irrelevant control mAb, and studied 6 or 24 hours later using bronchoalveolar lavage (BAL), histopathology, DNA nick-end-labeling assays, and electron microscopy. Normal mice treated with mAb Jo2 had significant increases in BAL protein at 6 hours, and BAL neutrophils at 24 hours, as compared to lpr mice and to mice treated with the irrelevant mAb. Neutrophil recruitment was preceded by increased mRNA expression for tumor necrosis factor-alpha, macrophage inflammatory protein-1alpha, macrophage inflammatory protein-2, macrophage chemotactic protein-1, and interleukin-6, but not interferon-gamma, transforming growth factor-ss, RANTES, eotaxin, or IP-10. Lung sections from Jo2-treated normal mice showed neutrophilic infiltrates, alveolar septal thickening, hemorrhage, and terminal dUTP nick-end-labeling-positive cells in the alveolar septae and airspaces. Type II pneumocyte apoptosis was confirmed by electron microscopy. Fas activation in vivo results in acute alveolar epithelial injury and lung inflammation, and may be important in the pathogenesis of acute lung injury.
Figures
Similar articles
-
Divergent Effects of Neutrophils on Fas-Induced Pulmonary Inflammation, Apoptosis, and Lung Damage.Shock. 2017 Feb;47(2):225-235. doi: 10.1097/SHK.0000000000000685. Shock. 2017. PMID: 27405068
-
The Fas/FasL pathway impairs the alveolar fluid clearance in mouse lungs.Am J Physiol Lung Cell Mol Physiol. 2013 Sep;305(5):L377-88. doi: 10.1152/ajplung.00271.2012. Epub 2013 Jun 28. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23812636 Free PMC article.
-
Recombinant human Fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits.Am J Physiol Lung Cell Mol Physiol. 2001 Aug;281(2):L328-35. doi: 10.1152/ajplung.2001.281.2.L328. Am J Physiol Lung Cell Mol Physiol. 2001. PMID: 11435207
-
The central role of Fas-ligand cell signaling in inflammatory lung diseases.J Cell Mol Med. 2004 Jul-Sep;8(3):285-93. doi: 10.1111/j.1582-4934.2004.tb00318.x. J Cell Mol Med. 2004. PMID: 15491504 Free PMC article. Review.
-
Science review: apoptosis in acute lung injury.Crit Care. 2003 Oct;7(5):355-8. doi: 10.1186/cc1861. Epub 2003 Apr 4. Crit Care. 2003. PMID: 12974968 Free PMC article. Review.
Cited by
-
Fas inhibition attenuates lipopolysaccharide-induced apoptosis and cytokine release of rat type II alveolar epithelial cells.Mol Biol Rep. 2010 Oct;37(7):3051-6. doi: 10.1007/s11033-009-9876-9. Epub 2009 Oct 13. Mol Biol Rep. 2010. PMID: 19823951
-
HB-EGF protects the lungs after intestinal ischemia/reperfusion injury.J Surg Res. 2010 Sep;163(1):86-95. doi: 10.1016/j.jss.2010.03.062. Epub 2010 Apr 24. J Surg Res. 2010. PMID: 20599214 Free PMC article.
-
Dimethylarginine dimethylaminohydrolase II overexpression attenuates LPS-mediated lung leak in acute lung injury.Am J Respir Cell Mol Biol. 2014 Mar;50(3):614-25. doi: 10.1165/rcmb.2013-0193OC. Am J Respir Cell Mol Biol. 2014. PMID: 24134589 Free PMC article.
-
Pathogenesis of indirect (secondary) acute lung injury.Expert Rev Respir Med. 2011 Feb;5(1):115-26. doi: 10.1586/ers.10.92. Expert Rev Respir Med. 2011. PMID: 21348592 Free PMC article. Review.
-
The Pla protease of Yersinia pestis degrades fas ligand to manipulate host cell death and inflammation.Cell Host Microbe. 2014 Apr 9;15(4):424-34. doi: 10.1016/j.chom.2014.03.005. Cell Host Microbe. 2014. PMID: 24721571 Free PMC article.
References
-
- Nagata S, Golstein P: The Fas death factor. Science 1995, 267:1449-1456 - PubMed
-
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S: The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66:233-243 - PubMed
-
- Itoh N, Nagata S: A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem 1993, 268:10932-10937 - PubMed
-
- Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P: Fas and perforin pathways as major mechanisms of T-cell mediated cytotoxicity. Science 1994, 265:528-530 - PubMed
-
- Liles WC, Klebanoff SJ: Regulation of apoptosis in neutrophils—Fas track to death? J Immunol 1995, 155:3289-3291 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

