Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor
- PMID: 11226287
- PMCID: PMC30186
- DOI: 10.1073/pnas.041609298
Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor
Abstract
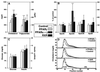
A common feature of many metabolic pathways is their control by retinoid X receptor (RXR) heterodimers. Dysregulation of such metabolic pathways can lead to the development of atherosclerosis, a disease influenced by both systemic and local factors. Here we analyzed the effects of activation of RXR and some of its heterodimers in apolipoprotein E -/- mice, a well established animal model of atherosclerosis. An RXR agonist drastically reduced the development of atherosclerosis. In addition, a ligand for the peroxisome proliferator-activated receptor (PPAR)gamma and a dual agonist of both PPARalpha and PPARgamma had moderate inhibitory effects. Both RXR and liver X receptor (LXR) agonists induced ATP-binding cassette protein 1 (ABC-1) expression and stimulated ABC-1-mediated cholesterol efflux from macrophages from wild-type, but not from LXRalpha and beta double -/-, mice. Hence, activation of ABC-1-mediated cholesterol efflux by the RXR/LXR heterodimer might contribute to the beneficial effects of rexinoids on atherosclerosis and warrant further evaluation of RXR/LXR agonists in prevention and treatment of atherosclerosis.
Figures

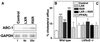
Similar articles
-
Activation of peroxisome proliferator-activated receptor gamma and retinoid X receptor results in net depletion of cellular cholesteryl esters in macrophages exposed to oxidized lipoproteins.Arterioscler Thromb Vasc Biol. 2003 Mar 1;23(3):475-82. doi: 10.1161/01.ATV.0000058860.62870.6E. Epub 2003 Jan 30. Arterioscler Thromb Vasc Biol. 2003. PMID: 12615696
-
Inhibition of liver x receptor/retinoid X receptor-mediated transcription contributes to the proatherogenic effects of arsenic in macrophages in vitro.Arterioscler Thromb Vasc Biol. 2010 Jun;30(6):1228-36. doi: 10.1161/ATVBAHA.110.205500. Epub 2010 Mar 25. Arterioscler Thromb Vasc Biol. 2010. PMID: 20339114
-
CD36-mediated cholesterol efflux is associated with PPARgamma activation via a MAPK-dependent COX-2 pathway in macrophages.Cardiovasc Res. 2009 Aug 1;83(3):457-64. doi: 10.1093/cvr/cvp118. Epub 2009 Apr 17. Cardiovasc Res. 2009. PMID: 19377069
-
Modulation of RXR function through ligand design.Biochim Biophys Acta. 2012 Jan;1821(1):57-69. doi: 10.1016/j.bbalip.2011.04.003. Epub 2011 Apr 16. Biochim Biophys Acta. 2012. PMID: 21515403 Review.
-
The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: From physiology to pathology.J Allergy Clin Immunol. 2013 Aug;132(2):264-86. doi: 10.1016/j.jaci.2013.05.044. J Allergy Clin Immunol. 2013. PMID: 23905916 Review.
Cited by
-
Thiazolidinedione-independent activation of peroxisome proliferator-activated receptor γ is a potential _target for diabetic macrovascular complications.J Diabetes Investig. 2012 Feb 20;3(1):11-23. doi: 10.1111/j.2040-1124.2011.00182.x. J Diabetes Investig. 2012. PMID: 24843540 Free PMC article. Review.
-
_targeting foam cell formation to improve recovery from ischemic stroke.Neurobiol Dis. 2023 Jun 1;181:106130. doi: 10.1016/j.nbd.2023.106130. Epub 2023 Apr 15. Neurobiol Dis. 2023. PMID: 37068641 Free PMC article. Review.
-
PDGF-induced proliferation of smooth muscular cells is related to the regulation of CREB phosphorylation and Nur77 expression.J Huazhong Univ Sci Technolog Med Sci. 2011 Apr;31(2):169-173. doi: 10.1007/s11596-011-0245-2. Epub 2011 Apr 20. J Huazhong Univ Sci Technolog Med Sci. 2011. PMID: 21505978
-
CNX-013-B2, a unique pan tissue acting rexinoid, modulates several nuclear receptors and controls multiple risk factors of the metabolic syndrome without risk of hypertriglyceridemia, hepatomegaly and body weight gain in animal models.Diabetol Metab Syndr. 2014 Aug 12;6(1):83. doi: 10.1186/1758-5996-6-83. eCollection 2014. Diabetol Metab Syndr. 2014. PMID: 25143786 Free PMC article.
-
Integrative pathway dissection of molecular mechanisms of moxLDL-induced vascular smooth muscle phenotype transformation.BMC Cardiovasc Disord. 2013 Jan 16;13:4. doi: 10.1186/1471-2261-13-4. BMC Cardiovasc Disord. 2013. PMID: 23324130 Free PMC article.
References
-
- Mangelsdorf D J, Evans R M. Cell. 1995;83:841–850. - PubMed
-
- Kastner P, Mark M, Chambon P. Cell. 1995;83:859–869. - PubMed
-
- Monczak Y, Trudel M, Lamph W W, Miller W H., Jr Blood. 1997;90:3345–3355. - PubMed
-
- Mukherjee R, Davies P A J, Crombie D L, Bischoff E D, Cesario R M, Jow L, Hamann L G, Boehm M F, Mondon C E, Nadzan A M, et al. Nature (London) 1997;386:407–410. - PubMed
-
- Peters J M, Hennuyer N, Staels B, Fruchart J C, Fievet C, Gonzalez F J, Auwerx J. J Biol Chem. 1997;272:27307–27312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

