Dual inactivation of RB and p53 pathways in RAS-induced melanomas
- PMID: 11238948
- PMCID: PMC86838
- DOI: 10.1128/MCB.21.6.2144-2153.2001
Dual inactivation of RB and p53 pathways in RAS-induced melanomas
Abstract
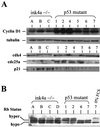
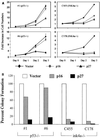
The frequent loss of both INK4a and ARF in melanoma raises the question of which INK4a-ARF gene product functions to suppress melanoma genesis in vivo. Moreover, the high incidence of INK4a-ARF inactivation in transformed melanocytes, along with the lack of p53 mutation, implies a cell type-specific role for INK4a-ARF that may not be complemented by other lesions of the RB and p53 pathways. A mouse model of cutaneous melanoma has been generated previously through the combined effects of INK4a(Delta2/3) deficiency (null for INK4a and ARF) and melanocyte-specific expression of activated RAS (tyrosinase-driven H-RAS(V12G), Tyr-RAS). In this study, we made use of this Tyr-RAS allele to determine whether activated RAS can cooperate with p53 loss in melanoma genesis, whether such melanomas are biologically comparable to those arising in INK4a(Delta2/3-/-) mice, and whether tumor-associated mutations emerge in the p16(INK4a)-RB pathway in such melanomas. Here, we report that p53 inactivation can cooperate with activated RAS to promote the development of cutaneous melanomas that are clinically indistinguishable from those arisen on the INK4a(Delta2/3) null background. Genomewide analysis of RAS-induced p53 mutant melanomas by comparative genomic hybridization and candidate gene surveys revealed alterations of key components governing RB-regulated G(1)/S transition, including c-Myc, cyclin D1, cdc25a, and p21(CIP1). Consistent with the profile of c-Myc dysregulation, the reintroduction of p16(INK4a) profoundly reduced the growth of Tyr-RAS INK4a(Delta2/3-/-) tumor cells but had no effect on tumor cells derived from Tyr-RAS p53(-/-) melanomas. Together, these data validate a role for p53 inactivation in melanomagenesis and suggest that both the RB and p53 pathways function to suppress melanocyte transformation in vivo in the mouse.
Figures







Similar articles
-
Genetic dissection of melanoma pathways in the mouse.Semin Cancer Biol. 2001 Jun;11(3):261-8. doi: 10.1006/scbi.2000.0376. Semin Cancer Biol. 2001. PMID: 11407950 Review.
-
Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence.Genes Dev. 2002 Nov 15;16(22):2923-34. doi: 10.1101/gad.1033002. Genes Dev. 2002. PMID: 12435633 Free PMC article.
-
Somatic p16(INK4a) loss accelerates melanomagenesis.Oncogene. 2010 Oct 28;29(43):5809-17. doi: 10.1038/onc.2010.314. Epub 2010 Aug 9. Oncogene. 2010. PMID: 20697345 Free PMC article.
-
Resistance of primary cultured mouse hepatic tumor cells to cellular senescence despite expression of p16(Ink4a), p19(Arf), p53, and p21(Waf1/Cip1).Mol Carcinog. 2001 Sep;32(1):9-18. doi: 10.1002/mc.1059. Mol Carcinog. 2001. PMID: 11568971
-
The INK4a/ARF tumor suppressor: one gene--two products--two pathways.Trends Biochem Sci. 1998 Aug;23(8):291-6. doi: 10.1016/s0968-0004(98)01236-5. Trends Biochem Sci. 1998. PMID: 9757829 Review.
Cited by
-
RB, p130 and p107 differentially repress G1/S and G2/M genes after p53 activation.Nucleic Acids Res. 2019 Dec 2;47(21):11197-11208. doi: 10.1093/nar/gkz961. Nucleic Acids Res. 2019. PMID: 31667499 Free PMC article.
-
Genetic analysis of Pten and Ink4a/Arf interactions in the suppression of tumorigenesis in mice.Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1455-60. doi: 10.1073/pnas.022632099. Epub 2002 Jan 29. Proc Natl Acad Sci U S A. 2002. PMID: 11818530 Free PMC article.
-
Growth factors and oncogenes as _targets in melanoma: lost in translation?Adv Dermatol. 2007;23:99-129. doi: 10.1016/j.yadr.2007.07.015. Adv Dermatol. 2007. PMID: 18159898 Free PMC article. Review.
-
Molecular pathways: _targeting NRAS in melanoma and acute myelogenous leukemia.Clin Cancer Res. 2014 Aug 15;20(16):4186-92. doi: 10.1158/1078-0432.CCR-13-3270. Epub 2014 Jun 3. Clin Cancer Res. 2014. PMID: 24895460 Free PMC article. Review.
-
Dissecting the contribution of p16(INK4A) and the Rb family to the Ras transformed phenotype.Mol Cell Biol. 2003 Apr;23(7):2530-42. doi: 10.1128/MCB.23.7.2530-2542.2003. Mol Cell Biol. 2003. PMID: 12640134 Free PMC article.
References
-
- Akslen L A, Monstad S E, Larsen B, Straume O, Ogreid D. Frequent mutations of the p53 gene in cutaneous melanoma of the nodular type. Int J Cancer. 1998;79:91–95. - PubMed
-
- Albino A P, Vidal M J, McNutt N S, Shea C R, Prieto V G, Nanus D M, Palmer J M, Hayward N K. Mutation and expression of the p53 gene in human malignant melanoma. Melanoma Res. 1994;4:35–45. - PubMed
-
- Bastian B C, LeBoit P E, Hamm H, Brocker E B, Pinkel D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 1998;58:2170–2175. - PubMed
-
- Berns K, Hijmans E M, Bernards R. Repression of c-Myc responsive genes in cycling cells causes G1 arrest through reduction of cyclin E/CDK2 kinase activity. Oncogene. 1997;15:1347–1356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
