Dual inactivation of RB and p53 pathways in RAS-induced melanomas
- PMID: 11238948
- PMCID: PMC86838
- DOI: 10.1128/MCB.21.6.2144-2153.2001
Dual inactivation of RB and p53 pathways in RAS-induced melanomas
Abstract
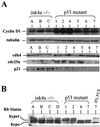
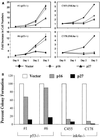
The frequent loss of both INK4a and ARF in melanoma raises the question of which INK4a-ARF gene product functions to suppress melanoma genesis in vivo. Moreover, the high incidence of INK4a-ARF inactivation in transformed melanocytes, along with the lack of p53 mutation, implies a cell type-specific role for INK4a-ARF that may not be complemented by other lesions of the RB and p53 pathways. A mouse model of cutaneous melanoma has been generated previously through the combined effects of INK4a(Delta2/3) deficiency (null for INK4a and ARF) and melanocyte-specific expression of activated RAS (tyrosinase-driven H-RAS(V12G), Tyr-RAS). In this study, we made use of this Tyr-RAS allele to determine whether activated RAS can cooperate with p53 loss in melanoma genesis, whether such melanomas are biologically comparable to those arising in INK4a(Delta2/3-/-) mice, and whether tumor-associated mutations emerge in the p16(INK4a)-RB pathway in such melanomas. Here, we report that p53 inactivation can cooperate with activated RAS to promote the development of cutaneous melanomas that are clinically indistinguishable from those arisen on the INK4a(Delta2/3) null background. Genomewide analysis of RAS-induced p53 mutant melanomas by comparative genomic hybridization and candidate gene surveys revealed alterations of key components governing RB-regulated G(1)/S transition, including c-Myc, cyclin D1, cdc25a, and p21(CIP1). Consistent with the profile of c-Myc dysregulation, the reintroduction of p16(INK4a) profoundly reduced the growth of Tyr-RAS INK4a(Delta2/3-/-) tumor cells but had no effect on tumor cells derived from Tyr-RAS p53(-/-) melanomas. Together, these data validate a role for p53 inactivation in melanomagenesis and suggest that both the RB and p53 pathways function to suppress melanocyte transformation in vivo in the mouse.
Figures







Similar articles
-
Genetic dissection of melanoma pathways in the mouse.Semin Cancer Biol. 2001 Jun;11(3):261-8. doi: 10.1006/scbi.2000.0376. Semin Cancer Biol. 2001. PMID: 11407950 Review.
-
Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence.Genes Dev. 2002 Nov 15;16(22):2923-34. doi: 10.1101/gad.1033002. Genes Dev. 2002. PMID: 12435633 Free PMC article.
-
Somatic p16(INK4a) loss accelerates melanomagenesis.Oncogene. 2010 Oct 28;29(43):5809-17. doi: 10.1038/onc.2010.314. Epub 2010 Aug 9. Oncogene. 2010. PMID: 20697345 Free PMC article.
-
Resistance of primary cultured mouse hepatic tumor cells to cellular senescence despite expression of p16(Ink4a), p19(Arf), p53, and p21(Waf1/Cip1).Mol Carcinog. 2001 Sep;32(1):9-18. doi: 10.1002/mc.1059. Mol Carcinog. 2001. PMID: 11568971
-
The INK4a/ARF tumor suppressor: one gene--two products--two pathways.Trends Biochem Sci. 1998 Aug;23(8):291-6. doi: 10.1016/s0968-0004(98)01236-5. Trends Biochem Sci. 1998. PMID: 9757829 Review.
Cited by
-
Ribosomal stress, p53 activation and the tanning response.Expert Rev Dermatol. 2008 Dec;3(6):649-656. doi: 10.1586/17469872.3.6.649. Expert Rev Dermatol. 2008. PMID: 22927886 Free PMC article.
-
Strain difference in transgene-induced tumorigenesis and suppressive effect of ionizing radiation.J Radiat Res. 2021 Jan 1;62(1):12-24. doi: 10.1093/jrr/rraa103. J Radiat Res. 2021. PMID: 33231252 Free PMC article.
-
MDM4 is a key therapeutic _target in cutaneous melanoma.Nat Med. 2012 Aug;18(8):1239-47. doi: 10.1038/nm.2863. Epub 2012 Jul 22. Nat Med. 2012. PMID: 22820643 Free PMC article.
-
A meta-analysis of transcriptome datasets characterizes malignant transformation from melanocytes and nevi to melanoma.Oncol Lett. 2018 Aug;16(2):1899-1911. doi: 10.3892/ol.2018.8861. Epub 2018 May 31. Oncol Lett. 2018. PMID: 30008882 Free PMC article.
-
New approaches to the biology of melanoma: a workshop of the National Institutes of Health Pathology B Study Section.Am J Pathol. 2002 Nov;161(5):1949-57. doi: 10.1016/S0002-9440(10)64470-7. Am J Pathol. 2002. PMID: 12414540 Free PMC article. No abstract available.
References
-
- Akslen L A, Monstad S E, Larsen B, Straume O, Ogreid D. Frequent mutations of the p53 gene in cutaneous melanoma of the nodular type. Int J Cancer. 1998;79:91–95. - PubMed
-
- Albino A P, Vidal M J, McNutt N S, Shea C R, Prieto V G, Nanus D M, Palmer J M, Hayward N K. Mutation and expression of the p53 gene in human malignant melanoma. Melanoma Res. 1994;4:35–45. - PubMed
-
- Bastian B C, LeBoit P E, Hamm H, Brocker E B, Pinkel D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 1998;58:2170–2175. - PubMed
-
- Berns K, Hijmans E M, Bernards R. Repression of c-Myc responsive genes in cycling cells causes G1 arrest through reduction of cyclin E/CDK2 kinase activity. Oncogene. 1997;15:1347–1356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
