The EXT1/EXT2 tumor suppressors: catalytic activities and role in heparan sulfate biosynthesis
- PMID: 11256613
- PMCID: PMC1083719
- DOI: 10.1093/embo-reports/kvd045
The EXT1/EXT2 tumor suppressors: catalytic activities and role in heparan sulfate biosynthesis
Abstract
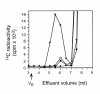
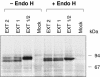
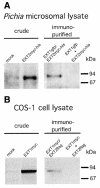
The D-glucuronyltransferase and N-acetyl-D-glucosaminyltransferase reactions in heparan sulfate biosynthesis have been associated with two genes, EXT1 and EXT2, which are also implicated in the inherited bone disorder, multiple exostoses. Since the cell systems used to express recombinant EXT proteins synthesize endogenous heparan sulfate, and the EXT proteins tend to associate, it has not been possible to define the functional roles of the individual protein species. We therefore expressed EXT1 and EXT2 in yeast, which does not synthesize heparan sulfate. The recombinant EXT1 and EXT2 were both found to catalyze both glycosyltransferase reactions in vitro. Coexpression of the two proteins, but not mixing of separately expressed recombinant EXT1 and EXT2, yields hetero-oligomeric complexes in yeast and mammalian cells, with augmented glycosyltransferase activities. This stimulation does not depend on the membrane-bound state of the proteins.
Figures

Similar articles
-
The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate.Proc Natl Acad Sci U S A. 2000 Jan 18;97(2):668-73. doi: 10.1073/pnas.97.2.668. Proc Natl Acad Sci U S A. 2000. PMID: 10639137 Free PMC article.
-
Contribution of EXT1, EXT2, and EXTL3 to heparan sulfate chain elongation.J Biol Chem. 2007 Nov 9;282(45):32802-10. doi: 10.1074/jbc.M703560200. Epub 2007 Aug 29. J Biol Chem. 2007. PMID: 17761672
-
rib-2, a Caenorhabditis elegans homolog of the human tumor suppressor EXT genes encodes a novel alpha1,4-N-acetylglucosaminyltransferase involved in the biosynthetic initiation and elongation of heparan sulfate.J Biol Chem. 2001 Feb 16;276(7):4834-8. doi: 10.1074/jbc.C000835200. Epub 2000 Dec 19. J Biol Chem. 2001. PMID: 11121397
-
Hereditary multiple exostoses and heparan sulfate polymerization.Biochim Biophys Acta. 2002 Dec 19;1573(3):346-55. doi: 10.1016/s0304-4165(02)00402-6. Biochim Biophys Acta. 2002. PMID: 12417417 Review.
-
Molecular basis of multiple exostoses: mutations in the EXT1 and EXT2 genes.Hum Mutat. 2000;15(3):220-7. doi: 10.1002/(SICI)1098-1004(200003)15:3<220::AID-HUMU2>3.0.CO;2-K. Hum Mutat. 2000. PMID: 10679937 Review.
Cited by
-
Reduced Expression of EXTL2, a Member of the Exostosin (EXT) Family of Glycosyltransferases, in Human Embryonic Kidney 293 Cells Results in Longer Heparan Sulfate Chains.J Biol Chem. 2015 May 22;290(21):13168-77. doi: 10.1074/jbc.M114.631754. Epub 2015 Mar 31. J Biol Chem. 2015. PMID: 25829497 Free PMC article.
-
Structural basis for heparan sulfate co-polymerase action by the EXT1-2 complex.Nat Chem Biol. 2023 May;19(5):565-574. doi: 10.1038/s41589-022-01220-2. Epub 2023 Jan 2. Nat Chem Biol. 2023. PMID: 36593275 Free PMC article.
-
Involvement of chondroitin sulfate synthase-3 (chondroitin synthase-2) in chondroitin polymerization through its interaction with chondroitin synthase-1 or chondroitin-polymerizing factor.Biochem J. 2007 May 1;403(3):545-52. doi: 10.1042/BJ20061876. Biochem J. 2007. PMID: 17253960 Free PMC article.
-
Loss of the Heparan Sulfate Proteoglycan Glypican5 Facilitates Long-Range Sonic Hedgehog Signaling.Stem Cells. 2019 Jul;37(7):899-909. doi: 10.1002/stem.3018. Epub 2019 May 13. Stem Cells. 2019. PMID: 30977233 Free PMC article.
-
Glycoconjugate glycosyltransferases.Glycoconj J. 2009 Nov;26(8):987-98. doi: 10.1007/s10719-008-9156-2. Glycoconj J. 2009. PMID: 18683045 Review.
References
-
- Ahn J., Ludecke, H.J., Lindow, S., Horton, W.A., Lee, B., Wagner, M.J., Horsthemke, B. and Wells, D.E. (1995) Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1). Nature Genet., 11, 137–143. - PubMed
-
- Gemmill T.R. and Trimble, R.B. (1999) Overview of N- and O-linked oligosaccharide structures found in various yeast species. Biochim. Biophys. Acta, 1426, 227–237. - PubMed
-
- Higgins D.R., Busser, K., Comiskey, J., Whittier, P.S., Purcell, T.J. and Hoeffler, J.P. (1998) Small vectors for expression based on dominant drug resistance with direct multicopy selection. Methods Mol. Biol., 103, 41–53. - PubMed
-
- Kitagawa H. and Paulson, J. (1994) Cloning of a novel alpha 2, 3-sialyltransferase that sialylates glycoprotein and glycolipid carbohydrate groups. J. Biol. Chem., 269, 1394–1401. - PubMed
-
- Kitagawa H., Shimakawa, H. and Sugahara, K. (1999) The tumor suppressor EXT-like gene EXTL2 encodes an α1,4-N-acetylhexosaminyltransferase that transfers N-acetylgalactosamine and N-acetylglucosamine to the common glycosaminoglycan-protein linkage region. The key enzyme for the chain initiation of heparan sulfate. J. Biol. Chem., 274, 13933–13937. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

