Protein unfolding by mitochondria. The Hsp70 import motor
- PMID: 11258479
- PMCID: PMC1083766
- DOI: 10.1093/embo-reports/kvd093
Protein unfolding by mitochondria. The Hsp70 import motor
Abstract
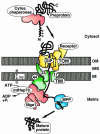
Protein unfolding is a key step in the import of some proteins into mitochondria and chloroplasts and in the degradation of regulatory proteins by ATP-dependent proteases. In contrast to protein folding, the reverse process has remained largely uninvestigated until now. This review discusses recent discoveries on the mechanism of protein unfolding during translocation into mitochondria. The mitochondria can actively unfold preproteins by unraveling them from the N-terminus. The central component of the mitochondrial import motor, the matrix heat shock protein 70, functions by both pulling and holding the preproteins.
Figures
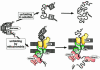
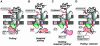



Similar articles
-
The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70.Cell. 1999 May 28;97(5):565-74. doi: 10.1016/s0092-8674(00)80768-0. Cell. 1999. PMID: 10367886
-
Differential requirement for the mitochondrial Hsp70-Tim44 complex in unfolding and translocation of preproteins.EMBO J. 1996 Jun 3;15(11):2668-77. EMBO J. 1996. PMID: 8654364 Free PMC article.
-
Unfolding of preproteins upon import into mitochondria.EMBO J. 1998 Nov 16;17(22):6497-507. doi: 10.1093/emboj/17.22.6497. EMBO J. 1998. PMID: 9822595 Free PMC article.
-
Protein import into mitochondria.Annu Rev Biochem. 1997;66:863-917. doi: 10.1146/annurev.biochem.66.1.863. Annu Rev Biochem. 1997. PMID: 9242927 Review.
-
The unfolding story of the Escherichia coli Hsp70 DnaK: is DnaK a holdase or an unfoldase?Mol Microbiol. 2002 Sep;45(5):1197-206. doi: 10.1046/j.1365-2958.2002.03093.x. Mol Microbiol. 2002. PMID: 12207689 Review.
Cited by
-
Spatial and temporal changes in Bax subcellular localization during anoikis.J Cell Biol. 2003 Aug 18;162(4):599-612. doi: 10.1083/jcb.200302154. J Cell Biol. 2003. PMID: 12925707 Free PMC article.
-
Autotransporters: The Cellular Environment Reshapes a Folding Mechanism to Promote Protein Transport.J Phys Chem Lett. 2012 Apr 2;3(8):1063-1071. doi: 10.1021/jz201654k. J Phys Chem Lett. 2012. PMID: 23687560 Free PMC article.
-
Transfer RNA travels from the cytoplasm to organelles.Wiley Interdiscip Rev RNA. 2011 Nov-Dec;2(6):802-17. doi: 10.1002/wrna.93. Epub 2011 Jul 11. Wiley Interdiscip Rev RNA. 2011. PMID: 21976284 Free PMC article. Review.
-
Plasmodium falciparum Hep1 Is Required to Prevent the Self Aggregation of PfHsp70-3.PLoS One. 2016 Jun 2;11(6):e0156446. doi: 10.1371/journal.pone.0156446. eCollection 2016. PLoS One. 2016. PMID: 27253881 Free PMC article.
-
The force exerted by the membrane potential during protein import into the mitochondrial matrix.Biophys J. 2004 Jun;86(6):3647-52. doi: 10.1529/biophysj.104.040865. Biophys J. 2004. PMID: 15189861 Free PMC article.
References
-
- Astumian R.D. (1997) Thermodynamics and kinetics of a Brownian motor. Science, 276, 917–922. - PubMed
-
- Bauer M.F., Hofmann, S., Neupert, W. and Brunner, M. (2000) Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol., 10, 25–31. - PubMed
-
- Bömer U., Meijer, M., Guiard, B., Dietmeier, K., Pfanner, N. and Rassow, J. (1997) The sorting route of cytochrome b2 branches from the general mitochondrial import pathway at the preprotein translocase of the inner membrane. J. Biol. Chem., 272, 30439–30446. - PubMed
-
- Bömer U., Maarse, A.C., Martin, F., Geissler, A., Merlin, A., Schönfisch, B., Meijer, M., Pfanner, N. and Rassow, J. (1998) Separation of structural and dynamic functions of the mitochondrial translocase: Tim44 is crucial for the inner membrane import sites in translocation of tightly folded domains, but not of loosely folded preproteins. EMBO J., 17, 4226–4237. - PMC - PubMed
-
- Branden C. and Tooze, J. (1998) Introduction to Protein Structure. Garland, New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

