Characterization of a late entry event in the replication cycle of human immunodeficiency virus type 2
- PMID: 11435571
- PMCID: PMC114419
- DOI: 10.1128/JVI.75.15.6914-6922.2001
Characterization of a late entry event in the replication cycle of human immunodeficiency virus type 2
Abstract
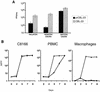
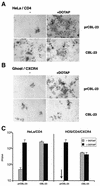
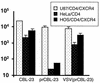
Certain human cell lines and primary macrophage cultures are restricted to infection by some primary isolates of human immunodeficiency virus type 2 (HIV-2), although early steps of the viral life cycle such as fusion at the plasma membrane and reverse transcription are fully supported. The late postintegration events, transcription, translation, assembly, budding, and maturation into infectious virions are functional in restrictive cells. Apart from primary macrophages, the restrictive cell types are actively dividing, and nuclear import of preintegration complexes (PICs) is not required for infection. We therefore postulate that the PICs are trapped in a cellular compartment, preventing subsequent steps in the replication cycle that lead to integration of the provirus. To test this we showed that HIV-2 particles pseudotyped with vesicular stomatitis virus envelope G protein, which delivers HIV into an endocytic compartment, could overcome the block to infection. We suggest that delivery of the viral core into an appropriate cellular compartment is a critical step during the entry process of HIV.
Figures

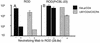


Similar articles
-
Lv2, a novel postentry restriction, is mediated by both capsid and envelope.J Virol. 2004 Feb;78(4):2006-16. doi: 10.1128/jvi.78.4.2006-2016.2004. J Virol. 2004. PMID: 14747565 Free PMC article.
-
Contribution of the C-terminal tri-lysine regions of human immunodeficiency virus type 1 integrase for efficient reverse transcription and viral DNA nuclear import.Retrovirology. 2005 Oct 18;2:62. doi: 10.1186/1742-4690-2-62. Retrovirology. 2005. PMID: 16232319 Free PMC article.
-
Tree Shrew Cells Transduced with Human CD4 and CCR5 Support Early Steps of HIV-1 Replication, but Viral Infectivity Is Restricted by APOBEC3.J Virol. 2021 Jul 26;95(16):e0002021. doi: 10.1128/JVI.00020-21. Epub 2021 Jul 26. J Virol. 2021. PMID: 34076481 Free PMC article.
-
Early stages of HIV replication: how to hijack cellular functions for a successful infection.AIDS Rev. 2004 Oct-Dec;6(4):199-207. AIDS Rev. 2004. PMID: 15700618 Review.
-
HIV-1 replication.Somat Cell Mol Genet. 2001 Nov;26(1-6):13-33. doi: 10.1023/a:1021070512287. Somat Cell Mol Genet. 2001. PMID: 12465460 Review.
Cited by
-
Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIVMAC/SIVSM/HIV-2 Lineage Prior to Integration.PLoS Pathog. 2015 Jul 16;11(7):e1005050. doi: 10.1371/journal.ppat.1005050. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26181333 Free PMC article.
-
A TRIM5alpha-independent post-entry restriction to HIV-1 infection of macaque cells that is dependent on the path of entry.Virology. 2007 Jul 5;363(2):310-8. doi: 10.1016/j.virol.2007.02.002. Epub 2007 Mar 9. Virology. 2007. PMID: 17350067 Free PMC article.
-
Early detection of a two-long-terminal-repeat junction molecule in the cytoplasm of recombinant murine leukemia virus-infected cells.J Virol. 2004 Jun;78(12):6190-9. doi: 10.1128/JVI.78.12.6190-6199.2004. J Virol. 2004. PMID: 15163712 Free PMC article.
-
An envelope-determined, pH-independent endocytic route of viral entry determines the susceptibility of human immunodeficiency virus type 1 (HIV-1) and HIV-2 to Lv2 restriction.J Virol. 2005 Aug;79(15):9410-8. doi: 10.1128/JVI.79.15.9410-9418.2005. J Virol. 2005. PMID: 16014904 Free PMC article.
-
Characterization of producer cell-dependent restriction of murine leukemia virus replication.J Virol. 2002 Jul;76(13):6609-17. doi: 10.1128/jvi.76.13.6609-6617.2002. J Virol. 2002. PMID: 12050374 Free PMC article.
References
-
- Akari H, Uchiyama T, Fukumori T, Iida S, Koyama A H, Adachi A. Pseudotyping human immunodeficiency virus type 1 by vesicular stomatitis virus G protein does not reduce the cell-dependent requirement of Vif for optimal infectivity: functional difference between Vif and Nef. J Gen Virol. 1999;80:2945–2959. - PubMed
-
- Best S, Le Tissier P, Towers G, Stoye J P. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature. 1996;382:826–829. - PubMed
-
- Binley J, Moore J P. HIV-cell fusion. The viral mousetrap. Nature. 1997;387:346–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

