Fas ligand triggers pulmonary silicosis
- PMID: 11457890
- PMCID: PMC2193452
- DOI: 10.1084/jem.194.2.155
Fas ligand triggers pulmonary silicosis
Abstract
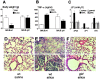
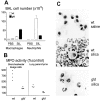
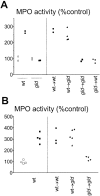
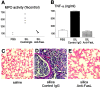
We investigated the role of Fas ligand in murine silicosis. Wild-type mice instilled with silica developed severe pulmonary inflammation, with local production of tumor necrosis factor (TNF)-alpha, and interstitial neutrophil and macrophage infiltration in the lungs. Strikingly, Fas ligand-deficient generalized lymphoproliferative disease mutant (gld) mice did not develop silicosis. The gld mice had markedly reduced neutrophil extravasation into bronchoalveolar space, and did not show increased TNF-alpha production, nor pulmonary inflammation. Bone marrow chimeras and local adoptive transfer demonstrated that wild-type, but not Fas ligand-deficient lung macrophages recruit neutrophils and initiate silicosis. Silica induced Fas ligand expression in lung macrophages in vitro and in vivo, and promoted Fas ligand-dependent macrophage apoptosis. Administration of neutralizing anti-Fas ligand antibody in vivo blocked induction of silicosis. Thus, Fas ligand plays a central role in induction of pulmonary silicosis.
Figures


Similar articles
-
Apoptosis underlies immunopathogenic mechanisms in acute silicosis.Am J Respir Cell Mol Biol. 2002 Jul;27(1):78-84. doi: 10.1165/ajrcmb.27.1.4717. Am J Respir Cell Mol Biol. 2002. PMID: 12091249
-
TNF contributes to the immunopathology of perforin/Fas ligand double deficiency.Immunol Cell Biol. 2002 Oct;80(5):436-40. doi: 10.1046/j.1440-1711.2002.01108.x. Immunol Cell Biol. 2002. PMID: 12225379
-
Resistance to acute silicosis in senescent rats: role of alveolar macrophages.Chem Res Toxicol. 2003 Dec;16(12):1520-7. doi: 10.1021/tx034139+. Chem Res Toxicol. 2003. PMID: 14680365
-
Autoimmune disease results from multiple interactive defects in apoptosis induction molecules and signaling pathways.Behring Inst Mitt. 1996 Oct;(97):200-19. Behring Inst Mitt. 1996. PMID: 8950477 Review.
-
Cellular interactions in the lpr and gld models of systemic autoimmunity.Adv Dent Res. 1996 Apr;10(1):76-80. doi: 10.1177/08959374960100011601. Adv Dent Res. 1996. PMID: 8934931 Review.
Cited by
-
Co-localization of iron binding on silica with p62/sequestosome1 (SQSTM1) in lung granulomas of mice with acute silicosis.J Clin Biochem Nutr. 2015 Jan;56(1):74-83. doi: 10.3164/jcbn.14-44. Epub 2014 Nov 28. J Clin Biochem Nutr. 2015. PMID: 25834305 Free PMC article.
-
Decidual macrophages are significantly increased in spontaneous miscarriages and over-express FasL: a potential role for macrophages in trophoblast apoptosis.Int J Mol Sci. 2012;13(7):9069-9080. doi: 10.3390/ijms13079069. Epub 2012 Jul 20. Int J Mol Sci. 2012. PMID: 22942752 Free PMC article.
-
The central role of Fas-ligand cell signaling in inflammatory lung diseases.J Cell Mol Med. 2004 Jul-Sep;8(3):285-93. doi: 10.1111/j.1582-4934.2004.tb00318.x. J Cell Mol Med. 2004. PMID: 15491504 Free PMC article. Review.
-
TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis.Am J Respir Cell Mol Biol. 2006 Mar;34(3):293-304. doi: 10.1165/rcmb.2005-0155OC. Epub 2005 Nov 4. Am J Respir Cell Mol Biol. 2006. PMID: 16272460 Free PMC article.
-
Implications of the Immune Landscape in COPD and Lung Cancer: Smoking Versus Other Causes.Front Immunol. 2022 Mar 21;13:846605. doi: 10.3389/fimmu.2022.846605. eCollection 2022. Front Immunol. 2022. PMID: 35386685 Free PMC article. Review.
References
-
- Weill H., Jones R.N., Parkes W.R. Silicosis and related diseases. In: Parkes W., editor. Occupational Lung Disorders. Butterworths; London: 1994. pp. 285–332.
-
- Mossman B.T., Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998;157:1666–1680. - PubMed
-
- Fujimura N. Pathology and pathophysiology of pneumoconiosis. Curr. Opin. Pulmon. Med. 2000;6:140–144. - PubMed
-
- Piguet P.F., Collart M.A., Grau G.E., Sappino A.P., Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990;344:245–247. - PubMed
-
- Nagata S. Fas ligand-induced apoptosis. Annu. Rev. Genet. 1999;33:29–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

