The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4
- PMID: 11485989
- PMCID: PMC312753
- DOI: 10.1101/gad.911501
The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4
Abstract
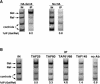
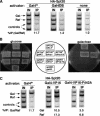
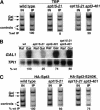
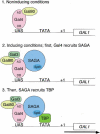
Previous studies demonstrated that the SAGA (Spt-Ada-Gcn5-Acetyltransferase) complex facilitates the binding of TATA-binding protein (TBP) during transcriptional activation of the GAL1 gene of Saccharomyces cerevisiae. TBP binding was shown to require the SAGA components Spt3 and Spt20/Ada5, but not the SAGA component Gcn5. We have now examined whether SAGA is directly required as a coactivator in vivo by using chromatin immunoprecipitation analysis. Our results demonstrate that SAGA is physically recruited in vivo to the upstream activation sequence (UAS) regions of the galactose-inducible GAL genes. This recruitment is dependent on both induction by galactose and the Gal4 activation domain. Furthermore, we demonstrate that another well-characterized activator, Gal4-VP16, also recruits SAGA in vivo. Finally, we provide evidence that a specific interaction between Spt3 and TBP in vivo is important for Gal4 transcriptional activation at a step after SAGA recruitment. These results, taken together with previous studies, demonstrate a dependent pathway for the recruitment of TBP to GAL gene promoters consisting of the recruitment of SAGA by Gal4 and the subsequent recruitment of TBP by SAGA.
Figures
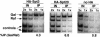
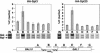
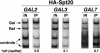

Similar articles
-
Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo.Mol Cell Biol. 2002 Nov;22(21):7365-71. doi: 10.1128/MCB.22.21.7365-7371.2002. Mol Cell Biol. 2002. PMID: 12370284 Free PMC article.
-
SAGA is an essential in vivo _target of the yeast acidic activator Gal4p.Genes Dev. 2001 Aug 1;15(15):1935-45. doi: 10.1101/gad.911401. Genes Dev. 2001. PMID: 11485988 Free PMC article.
-
The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo.Genes Dev. 1999 Nov 15;13(22):2940-5. doi: 10.1101/gad.13.22.2940. Genes Dev. 1999. PMID: 10580001 Free PMC article.
-
Recruitment of chromatin remodelling factors during gene activation via the glucocorticoid receptor N-terminal domain.Biochem Soc Trans. 2000;28(4):410-4. Biochem Soc Trans. 2000. PMID: 10961930 Review.
-
A SAGA of histone acetylation and gene expression.Trends Genet. 1997 Nov;13(11):427-9. doi: 10.1016/s0168-9525(97)01292-4. Trends Genet. 1997. PMID: 9385836 Review. No abstract available.
Cited by
-
The SAGA chromatin-modifying complex: the sum of its parts is greater than the whole.Genes Dev. 2020 Oct 1;34(19-20):1287-1303. doi: 10.1101/gad.341156.120. Genes Dev. 2020. PMID: 33004486 Free PMC article. Review.
-
Cellulases and beyond: the first 70 years of the enzyme producer Trichoderma reesei.Microb Cell Fact. 2016 Jun 10;15(1):106. doi: 10.1186/s12934-016-0507-6. Microb Cell Fact. 2016. PMID: 27287427 Free PMC article. Review.
-
Rsc4 connects the chromatin remodeler RSC to RNA polymerases.Mol Cell Biol. 2006 Jul;26(13):4920-33. doi: 10.1128/MCB.00415-06. Mol Cell Biol. 2006. PMID: 16782880 Free PMC article.
-
Mot3 is a transcriptional repressor of ergosterol biosynthetic genes and is required for normal vacuolar function in Saccharomyces cerevisiae.EMBO J. 2002 Aug 1;21(15):4114-24. doi: 10.1093/emboj/cdf415. EMBO J. 2002. PMID: 12145211 Free PMC article.
-
A _targeted histone acetyltransferase can create a sizable region of hyperacetylated chromatin and counteract the propagation of transcriptionally silent chromatin.Genetics. 2003 Sep;165(1):115-25. doi: 10.1093/genetics/165.1.115. Genetics. 2003. PMID: 14504221 Free PMC article.
References
-
- Barlev NA, Candau R, Wang L, Darpino P, Silverman N, Berger SL. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J Biol Chem. 1995;270:19337–19344. - PubMed
-
- Berger SL, Pina B, Silverman N, Marcus GA, Agapite J, Regier JL, Triezenberg SJ, Guarente L. Genetic isolation of ADA2: A potential transcriptional adaptor required for function of certain acidic activation domains. Cell. 1992;70:251–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
