Delta-Notch signaling and lateral inhibition in zebrafish spinal cord development
- PMID: 11495630
- PMCID: PMC37243
- DOI: 10.1186/1471-213x-1-13
Delta-Notch signaling and lateral inhibition in zebrafish spinal cord development
Abstract
Background: Vertebrate neural development requires precise coordination of cell proliferation and cell specification to guide orderly transition of mitotically active precursor cells into different types of post-mitotic neurons and glia. Lateral inhibition, mediated by the Delta-Notch signaling pathway, may provide a mechanism to regulate proliferation and specification in the vertebrate nervous system. We examined delta and notch gene expression in zebrafish embryos and tested the role of lateral inhibition in spinal cord patterning by ablating cells and genetically disrupting Delta-Notch signaling.
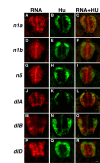
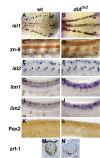
Results: Zebrafish embryos express multiple delta and notch genes throughout the developing nervous system. All or most proliferative precursors appeared to express notch genes whereas subsets of precursors and post-mitotic neurons expressed delta genes. When we ablated identified primary motor neurons soon after they were born, they were replaced, indicating that specified neurons laterally inhibit neighboring precursors. Mutation of a delta gene caused precursor cells of the trunk neural tube to cease dividing prematurely and develop as neurons. Additionally, mutant embryos had excess early specified neurons, with fates appropriate for their normal positions within the neural tube, and a concomitant deficit of late specified cells.
Conclusions: Our results are consistent with the idea that zebrafish Delta proteins, expressed by newly specified neurons, promote Notch activity in neighboring precursors. This signaling is required to maintain a proliferative precursor population and generate late-born neurons and glia. Thus, Delta-Notch signaling may diversify vertebrate neural cell fates by coordinating cell cycle control and cell specification.
Figures
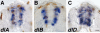


Similar articles
-
Oligodendrocyte specification in zebrafish requires notch-regulated cyclin-dependent kinase inhibitor function.J Neurosci. 2005 Jul 20;25(29):6836-44. doi: 10.1523/JNEUROSCI.0981-05.2005. J Neurosci. 2005. PMID: 16033893 Free PMC article.
-
Notch-regulated oligodendrocyte specification from radial glia in the spinal cord of zebrafish embryos.Dev Dyn. 2008 Aug;237(8):2081-9. doi: 10.1002/dvdy.21620. Dev Dyn. 2008. PMID: 18627107 Free PMC article.
-
Delta-Notch signaling regulates oligodendrocyte specification.Development. 2003 Aug;130(16):3747-55. doi: 10.1242/dev.00576. Development. 2003. PMID: 12835391
-
Delta-notch signaling and Drosophila cell fate choice.Dev Biol. 1994 Dec;166(2):415-30. doi: 10.1006/dbio.1994.1326. Dev Biol. 1994. PMID: 7813766 Review.
-
Notch signaling in the nervous system. Pieces still missing from the puzzle.Bioessays. 2000 Mar;22(3):264-73. doi: 10.1002/(SICI)1521-1878(200003)22:3<264::AID-BIES8>3.0.CO;2-M. Bioessays. 2000. PMID: 10684586 Review.
Cited by
-
Insights Into Central Nervous System Glial Cell Formation and Function From Zebrafish.Front Cell Dev Biol. 2021 Nov 29;9:754606. doi: 10.3389/fcell.2021.754606. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34912801 Free PMC article. Review.
-
Loss of CHSY1, a secreted FRINGE enzyme, causes syndromic brachydactyly in humans via increased NOTCH signaling.Am J Hum Genet. 2010 Dec 10;87(6):768-78. doi: 10.1016/j.ajhg.2010.11.005. Am J Hum Genet. 2010. PMID: 21129727 Free PMC article.
-
A revised model of Xenopus dorsal midline development: differential and separable requirements for Notch and Shh signaling.Dev Biol. 2011 Apr 15;352(2):254-66. doi: 10.1016/j.ydbio.2011.01.021. Epub 2011 Jan 27. Dev Biol. 2011. PMID: 21276789 Free PMC article.
-
Stomatal development in Arabidopsis.Arabidopsis Book. 2002;1:e0066. doi: 10.1199/tab.0066. Epub 2002 Sep 30. Arabidopsis Book. 2002. PMID: 22303215 Free PMC article.
-
Specification of CNS glia from neural stem cells in the embryonic neuroepithelium.Philos Trans R Soc Lond B Biol Sci. 2008 Jan 12;363(1489):71-85. doi: 10.1098/rstb.2006.2013. Philos Trans R Soc Lond B Biol Sci. 2008. PMID: 17282992 Free PMC article. Review.
References
-
- Edlund T, Jessell TM. Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell. 1999;96:211–224. - PubMed
-
- Greenwald I, Rubin GM. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992;68:271–281. - PubMed
-
- Raible DW, Eisen JS. Lateral specification of cell fate during vertebrate development. Curr Opin Genet Dev. 1995;5:444–449. - PubMed
-
- Doe CQ, Goodman CS. Early events in insect neurogenesis. II. The role of cell interactions and cell lineage in the determination of neuronal precursor cells. Dev Biol. 1985;111:206–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

