Pim serine/threonine kinases regulate the stability of Socs-1 protein
- PMID: 11854514
- PMCID: PMC122338
- DOI: 10.1073/pnas.042035699
Pim serine/threonine kinases regulate the stability of Socs-1 protein
Abstract
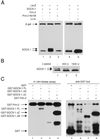
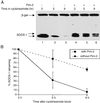
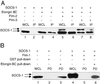
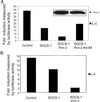
Studies of SOCS-1-deficient mice have implicated Socs-1 in the suppression of JAK-STAT (Janus tyrosine kinase-signal transducers and activators of transcription) signaling and T cell development. It has been suggested that the levels of Socs-1 protein may be regulated through the proteasome pathway. Here we show that Socs-1 interacts with members of the Pim family of serine/threonine kinases in thymocytes. Coexpression of the Pim kinases with Socs-1 results in phosphorylation and stabilization of the Socs-1 protein. The protein levels of Socs-1 are significantly reduced in the Pim-1(-/-), Pim-2(-/-) mice as compared with wild-type mice. Similar to Socs-1(-/-) mice, thymocytes from Pim-1(-/-), Pim-2(-/-) mice showed prolonged Stat6 phosphorylation upon IL-4 stimulation. These data suggest that the Pim kinases may regulate cytokine-induced JAK-STAT signaling through modulation of Socs-1 protein levels.
Figures

Similar articles
-
Suppressor of cytokine signaling (SOCS)-3 protein interacts with the insulin-like growth factor-I receptor.Biochem Biophys Res Commun. 2000 Nov 11;278(1):38-43. doi: 10.1006/bbrc.2000.3762. Biochem Biophys Res Commun. 2000. PMID: 11071852
-
Pim-1 kinase inhibits STAT5-dependent transcription via its interactions with SOCS1 and SOCS3.Blood. 2004 May 15;103(10):3744-50. doi: 10.1182/blood-2003-09-3126. Epub 2004 Feb 5. Blood. 2004. PMID: 14764533
-
IL-4 signaling is regulated through the recruitment of phosphatases, kinases, and SOCS proteins to the receptor complex.Cold Spring Harb Symp Quant Biol. 1999;64:405-16. doi: 10.1101/sqb.1999.64.405. Cold Spring Harb Symp Quant Biol. 1999. PMID: 11232315 Review. No abstract available.
-
SHP-2 regulates SOCS-1-mediated Janus kinase-2 ubiquitination/degradation downstream of the prolactin receptor.J Biol Chem. 2003 Dec 26;278(52):52021-31. doi: 10.1074/jbc.M306758200. Epub 2003 Oct 1. J Biol Chem. 2003. PMID: 14522994
-
SOCS proteins: negative regulators of cytokine signaling.Stem Cells. 2001;19(5):378-87. doi: 10.1634/stemcells.19-5-378. Stem Cells. 2001. PMID: 11553846 Review.
Cited by
-
SOCS1 and SOCS3 as key checkpoint molecules in the immune responses associated to skin inflammation and malignant transformation.Front Immunol. 2024 Jun 21;15:1393799. doi: 10.3389/fimmu.2024.1393799. eCollection 2024. Front Immunol. 2024. PMID: 38975347 Free PMC article. Review.
-
SOCS-JAK-STAT inhibitors and SOCS mimetics as treatment options for autoimmune uveitis, psoriasis, lupus, and autoimmune encephalitis.Front Immunol. 2023 Oct 26;14:1271102. doi: 10.3389/fimmu.2023.1271102. eCollection 2023. Front Immunol. 2023. PMID: 38022642 Free PMC article. Review.
-
Pim Kinases: Important Regulators of Cardiovascular Disease.Int J Mol Sci. 2023 Jul 18;24(14):11582. doi: 10.3390/ijms241411582. Int J Mol Sci. 2023. PMID: 37511341 Free PMC article. Review.
-
_targeting Pim kinases in hematological cancers: molecular and clinical review.Mol Cancer. 2023 Jan 25;22(1):18. doi: 10.1186/s12943-023-01721-1. Mol Cancer. 2023. PMID: 36694243 Free PMC article. Review.
-
_targeting PIM Kinases to Improve the Efficacy of Immunotherapy.Cells. 2022 Nov 21;11(22):3700. doi: 10.3390/cells11223700. Cells. 2022. PMID: 36429128 Free PMC article. Review.
References
-
- Leonard W J, O'Shea J J. Annu Rev Immunol. 1998;16:293–322. - PubMed
-
- Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, et al. Nature (London) 1997;387:921–924. - PubMed
-
- Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, et al. Nature (London) 1997;387:924–929. - PubMed
-
- Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, Hilton D J. Nature (London) 1997;387:917–921. - PubMed
-
- Chen X P, Losman J A, Rothman P. Immunity. 2000;13:287–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

