Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H(2)O(2), Fe(2+), and Mn(2+)
- PMID: 12029030
- PMCID: PMC135095
- DOI: 10.1128/JB.184.12.3151-3158.2002
Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H(2)O(2), Fe(2+), and Mn(2+)
Abstract
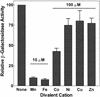
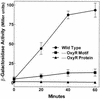
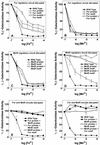
MntH, a bacterial homolog of mammalian natural resistance associated macrophage protein 1 (Nramp1), is a primary transporter for Mn(2+) influx in Salmonella enterica serovar Typhimurium and Escherichia coli. S. enterica serovar Typhimurium MntH contributes to H(2)O(2) resistance and is important for full virulence. Consistent with its phenotype and function, mntH is regulated at the transcriptional level by both H(2)O(2) and substrate cation. We have now identified three trans-acting regulatory factors and the three corresponding cis-acting mntH promoter motifs that mediate this regulation. In the presence of hydrogen peroxide, mntH is activated by OxyR, acting through an OxyR-binding motif centered just upstream of the likely -35 RNA polymerase-binding site. In the presence of Fe(2+), mntH is repressed primarily by Fur, acting through a Fur-binding motif overlapping the -35 region. In the presence of Mn(2+), mntH is repressed primarily by the Salmonella equivalent of E. coli b0817, a distant homolog of the Bacillus subtilis manganese transport repressor, MntR, acting through an inverted-repeat motif located between the likely -10 polymerase binding site and the ribosome binding site. E. coli b0817 was recently shown to bind the identical inverted-repeat motif in the E. coli mntH promoter and hence has been renamed MntR (S. I. Patzer and K. Hantke, J. Bacteriol. 183:4806-4813, 2001). Using Deltafur, DeltamntR, and Deltafur DeltamntR mutant strains as well as mutations in the Fur- and MntR-binding motif elements, we found that Fe(2+) can also mediate repression through the Mn(2+) repressor MntR.
Figures

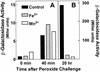
Similar articles
-
SitABCD is the alkaline Mn(2+) transporter of Salmonella enterica serovar Typhimurium.J Bacteriol. 2002 Jun;184(12):3159-66. doi: 10.1128/JB.184.12.3159-3166.2002. J Bacteriol. 2002. PMID: 12029031 Free PMC article.
-
Transcriptional regulation of sitABCD of Salmonella enterica serovar Typhimurium by MntR and Fur.J Bacteriol. 2005 Feb;187(3):912-22. doi: 10.1128/JB.187.3.912-922.2005. J Bacteriol. 2005. PMID: 15659669 Free PMC article.
-
Dual repression by Fe(2+)-Fur and Mn(2+)-MntR of the mntH gene, encoding an NRAMP-like Mn(2+) transporter in Escherichia coli.J Bacteriol. 2001 Aug;183(16):4806-13. doi: 10.1128/JB.183.16.4806-4813.2001. J Bacteriol. 2001. PMID: 11466284 Free PMC article.
-
Manganese Transporter Proteins in Salmonella enterica serovar Typhimurium.J Microbiol. 2023 Mar;61(3):289-296. doi: 10.1007/s12275-023-00027-7. Epub 2023 Mar 2. J Microbiol. 2023. PMID: 36862278 Review.
-
The OxyR regulon.Antonie Van Leeuwenhoek. 1990 Oct;58(3):157-61. doi: 10.1007/BF00548927. Antonie Van Leeuwenhoek. 1990. PMID: 2256675 Review.
Cited by
-
Superoxide poisons mononuclear iron enzymes by causing mismetallation.Mol Microbiol. 2013 Jul;89(1):123-34. doi: 10.1111/mmi.12263. Epub 2013 Jun 7. Mol Microbiol. 2013. PMID: 23678969 Free PMC article.
-
SitABCD is the alkaline Mn(2+) transporter of Salmonella enterica serovar Typhimurium.J Bacteriol. 2002 Jun;184(12):3159-66. doi: 10.1128/JB.184.12.3159-3166.2002. J Bacteriol. 2002. PMID: 12029031 Free PMC article.
-
A non-redundant microarray of genes for two related bacteria.Nucleic Acids Res. 2003 Apr 1;31(7):1869-76. doi: 10.1093/nar/gkg298. Nucleic Acids Res. 2003. PMID: 12655003 Free PMC article.
-
Curcumin increases the pathogenicity of Salmonella enterica serovar Typhimurium in murine model.PLoS One. 2010 Jul 9;5(7):e11511. doi: 10.1371/journal.pone.0011511. PLoS One. 2010. PMID: 20634977 Free PMC article.
-
Disruption of Glycolysis by Nutritional Immunity Activates a Two-Component System That Coordinates a Metabolic and Antihost Response by Staphylococcus aureus.mBio. 2019 Aug 6;10(4):e01321-19. doi: 10.1128/mBio.01321-19. mBio. 2019. PMID: 31387906 Free PMC article.
References
-
- Althaus, E. W., C. E. Outten, K. E. Olson, H. Cao, and T. V. O'Halloran. 1999. The ferric uptake regulation (Fur) repressor is a zinc metalloprotein. Biochemistry 38:6559-6569. - PubMed
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Atkinson, P. G., and C. H. Barton. 1998. Ectopic expression of Nramp1 in COS-1 cells modulates iron accumulation. FEBS Lett. 425:239-242. - PubMed
-
- Bearden, S. W., and R. D. Perry. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32:403-414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

