The yeast prion Ure2p retains its native alpha-helical conformation upon assembly into protein fibrils in vitro
- PMID: 12065404
- PMCID: PMC126058
- DOI: 10.1093/emboj/cdf303
The yeast prion Ure2p retains its native alpha-helical conformation upon assembly into protein fibrils in vitro
Abstract
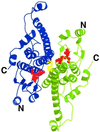
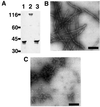
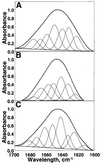
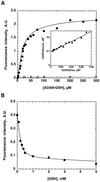
The yeast inheritable phenotype [URE3] is thought to result from conformational changes in the normally soluble and highly helical protein Ure2p. In vitro, the protein spontaneously forms long, straight, insoluble protein fibrils at neutral pH. Here we show that fibrils of intact Ure2p assembled in vitro do not possess the cross beta-structure of amyloid, but instead are formed by the polymerization of native-like helical subunits that retain the ability to bind substrate analogues. We further show that dissociation of the normally dimeric protein to its constituent monomers is a prerequisite for assembly into fibrils. By analysing the nature of early assembly intermediates, as well as fully assembled Ure2p fibrils using atomic force microscopy, and combining the results with experiments that probe the fidelity of the native fold in protein fibrils, we present a model for fibril formation, based on assembly of native-like monomers, driven by interactions between the N-terminal glutamine and asparagine-rich region and the C-terminal functional domain. The results provide a rationale for the effect of mutagenesis on prion formation and new insights into the mechanism by which this, and possibly other inheritable factors, can be propagated.
Figures



Similar articles
-
Structure of the prion Ure2p in protein fibrils assembled in vitro.J Biol Chem. 2005 Nov 4;280(44):37149-58. doi: 10.1074/jbc.M506917200. Epub 2005 Aug 30. J Biol Chem. 2005. PMID: 16131495
-
Structural characterization of the fibrillar form of the yeast Saccharomyces cerevisiae prion Ure2p.Biochemistry. 2004 May 4;43(17):5022-32. doi: 10.1021/bi049828e. Biochemistry. 2004. PMID: 15109261
-
The native-like conformation of Ure2p in fibrils assembled under physiologically relevant conditions switches to an amyloid-like conformation upon heat-treatment of the fibrils.J Struct Biol. 2003 Feb;141(2):132-42. doi: 10.1016/s1047-8477(02)00606-8. J Struct Biol. 2003. PMID: 12615539
-
The yeast prion protein Ure2: insights into the mechanism of amyloid formation.Biochem Soc Trans. 2011 Oct;39(5):1359-64. doi: 10.1042/BST0391359. Biochem Soc Trans. 2011. PMID: 21936815 Review.
-
Prion amyloid structure explains templating: how proteins can be genes.FEMS Yeast Res. 2010 Dec;10(8):980-91. doi: 10.1111/j.1567-1364.2010.00666.x. FEMS Yeast Res. 2010. PMID: 20726897 Free PMC article. Review.
Cited by
-
Self-assembled amyloid-like oligomeric-cohesin Scaffoldin for augmented protein display on the saccharomyces cerevisiae cell surface.Appl Environ Microbiol. 2012 May;78(9):3249-55. doi: 10.1128/AEM.07745-11. Epub 2012 Feb 17. Appl Environ Microbiol. 2012. PMID: 22344635 Free PMC article.
-
Ure2, a prion precursor with homology to glutathione S-transferase, protects Saccharomyces cerevisiae cells from heavy metal ion and oxidant toxicity.J Biol Chem. 2003 Apr 11;278(15):12826-33. doi: 10.1074/jbc.M212186200. Epub 2003 Jan 31. J Biol Chem. 2003. PMID: 12562760 Free PMC article.
-
The Role of Functional Amyloids in Multicellular Growth and Development of Gram-Positive Bacteria.Biomolecules. 2017 Aug 7;7(3):60. doi: 10.3390/biom7030060. Biomolecules. 2017. PMID: 28783117 Free PMC article. Review.
-
Structural Analyses of Designed α-Helix and β-Sheet Peptide Nanofibers Using Solid-State Nuclear Magnetic Resonance and Cryo-Electron Microscopy and Introduction of Structure-Based Metal-Responsive Properties.Int J Mol Sci. 2024 Jan 16;25(2):1111. doi: 10.3390/ijms25021111. Int J Mol Sci. 2024. PMID: 38256184 Free PMC article.
-
Prions in yeast.Genetics. 2012 Aug;191(4):1041-72. doi: 10.1534/genetics.111.137760. Genetics. 2012. PMID: 22879407 Free PMC article. Review.
References
-
- Board P.G. et al. (2000) Identification, characterization and crystal structure of the omega class glutathione transferases. J. Biol. Chem., 275, 24798–24806. - PubMed
-
- Bousset L., Belrhali,H., Janin,J., Melki,R. and Morera,S. (2001a) Structure of the globular region of the prion protein Ure2 from the yeast Saccharomyces cerevisiae. Structure, 9, 39–46. - PubMed
-
- Bousset L., Belrhali,H., Melki,R. and Morera,S. (2001b) Crystal structures of the yeast prion Ure2p functional region in complex with glutathione and related compounds. Biochemistry, 40, 13564–13573. - PubMed
-
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem., 72, 248–254. - PubMed
-
- Carrell R.W. and Gooptu,B. (1998) Conformational changes and disease—serpins, prions and Alzheimer’s. Curr. Opin. Struct. Biol., 8, 799–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

