Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia
- PMID: 12377750
- PMCID: PMC4027051
- DOI: 10.1161/01.atv.0000033090.81345.e6
Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia
Abstract
Objective: The adipocyte fatty acid-binding protein, aP2, has important effects on insulin resistance, lipid metabolism, and atherosclerosis. Its expression in macrophages enhances early foam cell formation and atherosclerosis in vivo. This study was designed to determine whether aP2 deficiency has a similar effect in the setting of advanced atherosclerosis and severe hypercholesterolemia.
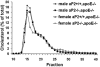
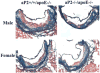
Methods and results: Mice deficient in aP2 and apolipoprotein E (aP2(-/-)apoE(-/-) mice) and apolipoprotein E-deficient control mice (apoE(-/-) mice) were fed a Western diet for 14 weeks. No significant differences in fasting serum levels of cholesterol, triglycerides, or free fatty acids were found between groups for each sex. Compared with apoE(-/-) control mice, male and female aP2(-/-)apoE(-/-) mice had significant reductions in mean atherosclerotic lesion size in the proximal aorta, en face aorta, and innominate/right carotid artery. Feeding the Western diet in the apoE-deficient background did not cause a significant reduction in insulin sensitivity in vivo, as determined by steady-state serum glucose levels and insulin tolerance testing.
Conclusions: These data demonstrate an important role for aP2 expression in the advanced stages of atherosclerotic lesion formation. Thus, aP2 provides an important physiological link between different features of the metabolic syndrome and is a potential _target for therapy of atherosclerosis.
Figures
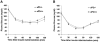
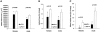

Similar articles
-
Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice.Circulation. 2004 Sep 14;110(11):1492-8. doi: 10.1161/01.CIR.0000141735.13202.B6. Epub 2004 Sep 7. Circulation. 2004. PMID: 15353487 Free PMC article.
-
Role of macrophage-expressed adipocyte fatty acid binding protein in the development of accelerated atherosclerosis in hypercholesterolemic mice.FASEB J. 2001 Dec;15(14):2733-5. doi: 10.1096/fj.01-0374fje. Epub 2001 Oct 15. FASEB J. 2001. PMID: 11606480
-
Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis.Nat Med. 2001 Jun;7(6):699-705. doi: 10.1038/89076. Nat Med. 2001. PMID: 11385507 Free PMC article.
-
Macrophages, inflammation, and atherosclerosis.Int J Obes Relat Metab Disord. 2003 Dec;27 Suppl 3:S35-40. doi: 10.1038/sj.ijo.0802498. Int J Obes Relat Metab Disord. 2003. PMID: 14704742 Review.
-
Cytoplasmic fatty acid-binding proteins: emerging roles in metabolism and atherosclerosis.Curr Opin Lipidol. 2002 Apr;13(2):141-7. doi: 10.1097/00041433-200204000-00005. Curr Opin Lipidol. 2002. PMID: 11891416 Review.
Cited by
-
Adipose inflammation at the heart of vascular disease.Clin Sci (Lond). 2016 Nov 1;130(22):2101-2104. doi: 10.1042/CS20160628. Clin Sci (Lond). 2016. PMID: 27729474 Free PMC article.
-
Regulation of macrophage functions by FABP-mediated inflammatory and metabolic pathways.Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Aug;1866(8):158964. doi: 10.1016/j.bbalip.2021.158964. Epub 2021 May 10. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 33984518 Free PMC article. Review.
-
Receptor mediated elevation in FABP4 levels by advanced glycation end products induces cholesterol and triacylglycerol accumulation in THP-1 macrophages.Lipids. 2011 Jun;46(6):479-86. doi: 10.1007/s11745-011-3542-4. Epub 2011 Feb 20. Lipids. 2011. PMID: 21336983
-
Carnitine transport and fatty acid oxidation.Biochim Biophys Acta. 2016 Oct;1863(10):2422-35. doi: 10.1016/j.bbamcr.2016.01.023. Epub 2016 Jan 29. Biochim Biophys Acta. 2016. PMID: 26828774 Free PMC article. Review.
-
Identification of new susceptibility loci for type 2 diabetes and shared etiological pathways with coronary heart disease.Nat Genet. 2017 Oct;49(10):1450-1457. doi: 10.1038/ng.3943. Epub 2017 Sep 4. Nat Genet. 2017. PMID: 28869590 Free PMC article.
References
-
- Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta. 2000;1486:28–44. - PubMed
-
- Coe NR, Bernlohr DA. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim Biophys Acta. 1998;1391:287–306. - PubMed
-
- Distel RJ, Robinson GS, Spiegelman BM. Fatty acid regulation of gene expression: transcriptional and post-transcriptional mechanisms. J Biol Chem. 1992;267:5937–5941. - PubMed
-
- Chaput E, Saladin R, Silvestre M, Edgar AD. Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochem Biophys Res Commun. 2000;271:445–450. - PubMed
-
- Melki SA, Abumrad NA. Expression of the adipocyte fatty acid-binding protein in streptozotocin-diabetes: effects of insulin deficiency and supplementation. J Lipid Res. 1993;34:1527–1534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

