Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture
- PMID: 12499198
- PMCID: PMC149013
- DOI: 10.1128/AAC.47.1.244-254.2003
Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture
Abstract
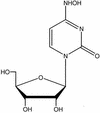
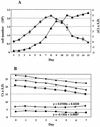
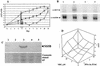
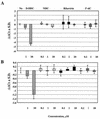
A base-modified nucleoside analogue, beta-D-N(4)-hydroxycytidine (NHC), was found to have antipestivirus and antihepacivirus activities. This compound inhibited the production of cytopathic bovine viral diarrhea virus (BVDV) RNA in a dose-dependant manner with a 90% effective concentration (EC(90)) of 5.4 microM, an observation that was confirmed by virus yield assays (EC(90) = 2 microM). When tested for hepatitis C virus (HCV) replicon RNA reduction in Huh7 cells, NHC had an EC(90) of 5 microM on day 4. The HCV RNA reduction was incubation time and nucleoside concentration dependent. The in vitro antiviral effect of NHC was additive with recombinant alpha interferon-2a and could be prevented by the addition of exogenous cytidine and uridine but not of other natural ribo- or 2'-deoxynucleosides. When HCV RNA replicon cells were cultured in the presence of increasing concentrations of NHC (up to 40 micro M) for up to 45 cell passages, no resistant replicon was selected. Similarly, resistant BVDV could not be selected after 20 passages. NHC was phosphorylated to the triphosphate form in Huh7 cells, but in cell-free HCV NS5B assays, synthetic NHC-triphosphate (NHC-TP) did not inhibit the polymerization reaction. Instead, NHC-TP appeared to serve as a weak alternative substrate for the viral polymerase, thereby changing the mobility of the product in polyacrylamide electrophoresis gels. We speculate that incorporated nucleoside analogues with the capacity of changing the thermodynamics of regulatory secondary structures (with or without introducing mutations) may represent an important class of new antiviral agents for the treatment of RNA virus infections, especially HCV.
Figures




Similar articles
-
Establishment of a subgenomic replicon for bovine viral diarrhea virus in Huh-7 cells and modulation of interferon-regulated factor 3-mediated antiviral response.J Virol. 2005 Mar;79(5):2788-96. doi: 10.1128/JVI.79.5.2788-2796.2005. J Virol. 2005. PMID: 15708997 Free PMC article.
-
Synthesis of beta-enantiomers of N4-hydroxy-3'-deoxypyrimidine nucleosides and their evaluation against bovine viral diarrhoea virus and hepatitis C virus in cell culture.Antivir Chem Chemother. 2004 Jan;15(1):43-55. doi: 10.1177/095632020401500105. Antivir Chem Chemother. 2004. PMID: 15074714
-
Small-Molecule Antiviral β-d-N4-Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance.J Virol. 2019 Nov 26;93(24):e01348-19. doi: 10.1128/JVI.01348-19. Print 2019 Dec 15. J Virol. 2019. PMID: 31578288 Free PMC article.
-
Bovine viral diarrhea virus as a surrogate model of hepatitis C virus for the evaluation of antiviral agents.Antiviral Res. 2003 Sep;60(1):1-15. doi: 10.1016/s0166-3542(03)00174-8. Antiviral Res. 2003. PMID: 14516916 Review.
-
What is known about the antiviral agents active against bovine viral diarrhea virus (BVDV)?Curr Med Chem. 2010;17(26):2933-55. doi: 10.2174/092986710792065036. Curr Med Chem. 2010. PMID: 20858174 Review.
Cited by
-
Synthesis, evaluation of anti-HIV-1 and anti-HCV activity of novel 2',3'-dideoxy-2',2'-difluoro-4'-azanucleosides.Bioorg Med Chem. 2012 Dec 1;20(23):6885-93. doi: 10.1016/j.bmc.2012.09.026. Epub 2012 Sep 23. Bioorg Med Chem. 2012. PMID: 23085031 Free PMC article.
-
COVID-19 Therapeutic Options Under Investigation.Front Pharmacol. 2020 Aug 6;11:1196. doi: 10.3389/fphar.2020.01196. eCollection 2020. Front Pharmacol. 2020. PMID: 32848795 Free PMC article. Review.
-
Correction: Reynard, O.; et al. Identification of a New Ribonucleoside Inhibitor of Ebola Virus Replication. Viruses 2015, 7, 6233‒6240.Viruses. 2016 May 18;8(5):137. doi: 10.3390/v8050137. Viruses. 2016. PMID: 27213424 Free PMC article.
-
Synthesis and anti-hepatitis B virus and anti-hepatitis C virus activities of 7-deazaneplanocin A analogues in vitro.J Med Chem. 2009 Jan 8;52(1):206-13. doi: 10.1021/jm801418v. J Med Chem. 2009. PMID: 19072694 Free PMC article.
-
Synthesis of carbocyclic nucleoside analogs with five-membered heterocyclic nucleobases.Tetrahedron Lett. 2015 Jun 3;56(23):3587-3590. doi: 10.1016/j.tetlet.2015.01.051. Tetrahedron Lett. 2015. PMID: 26028788 Free PMC article.
References
-
- Alt, M., S. Eisenhardt, M. Serwe, R. Renz, J. W. Engels, and W. H. Caselmann. 1999. Comparative inhibitory potential of differently modified antisense oligodeoxynucleotides on hepatitis C virus translation. Eur. J. Clin. Investig. 29:868-876. - PubMed
-
- Alter, M. J., D. Kruszon-Moran, O. V. Nainan, G. M. McQuillan, F. Gao, L. A. Moyer, R. A. Kaslow, and H. S. Margolis. 1999. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N. Engl. J. Med. 341:556-562. - PubMed
-
- Bartenschlager, R., and V. Lohmann. 2000. Replication of hepatitis C virus. J. Gen. Virol. 81:1631-1648. - PubMed
-
- Belen'kii, M. S., and R. F. Schinazi. 1994. Multiple drug effect analysis with confidence interval. Antivir. Res. 25:1-11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

