Dissecting the contribution of p16(INK4A) and the Rb family to the Ras transformed phenotype
- PMID: 12640134
- PMCID: PMC150721
- DOI: 10.1128/MCB.23.7.2530-2542.2003
Dissecting the contribution of p16(INK4A) and the Rb family to the Ras transformed phenotype
Abstract
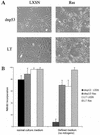
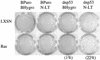
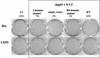
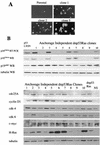
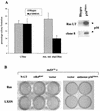
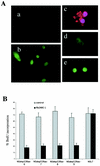
Although oncogenic Ras commonly contributes to the development of cancer, in normal primary cells it induces cell cycle arrest rather than transformation. Here we analyze the additional genetic changes required for Ras to promote cell cycle progression rather than arrest. We show that loss of p53 is sufficient for oncogenic Ras to stimulate proliferation in the absence of extrinsic mitogens in attached cells. However, surprisingly, we find that p53 loss is not sufficient for Ras to overcome anchorage dependence or contact inhibition. In contrast, expression of simian virus 40 (SV40) large T antigen (LT) allows Ras to overcome these additional cell cycle controls. Mutational analysis of SV40 LT shows that this action of SV40 LT depends on its ability to inactivate the retinoblastoma (Rb) family of proteins, in concert with the loss of p53. Importantly, we show that inactivation of the Rb family of proteins can be mimicked by loss of the cyclin-dependent kinase inhibitor p16(INK4A). p16(INK4A) is commonly lost in human tumors, but its contribution to the transformed phenotype is unknown. We demonstrate here a role for p16(INK4A) in the loss of cell cycle controls required for tumorigenesis and show how accumulating genetic changes cooperate and contribute to the transformed phenotype.
Figures

Similar articles
-
Loss of p19(ARF) eliminates the requirement for the pRB-binding motif in simian virus 40 large T antigen-mediated transformation.Mol Cell Biol. 2000 Oct;20(20):7624-33. doi: 10.1128/MCB.20.20.7624-7633.2000. Mol Cell Biol. 2000. PMID: 11003658 Free PMC article.
-
Dual inactivation of RB and p53 pathways in RAS-induced melanomas.Mol Cell Biol. 2001 Mar;21(6):2144-53. doi: 10.1128/MCB.21.6.2144-2153.2001. Mol Cell Biol. 2001. PMID: 11238948 Free PMC article.
-
E2FBP1 antagonizes the p16(INK4A)-Rb tumor suppressor machinery for growth suppression and cellular senescence by regulating promyelocytic leukemia protein stability.Int J Oral Sci. 2011 Oct;3(4):200-8. doi: 10.4248/IJOS11071. Int J Oral Sci. 2011. PMID: 22010578 Free PMC article.
-
Unraveling human tumor suppressor pathways: a tale of the INK4A locus.Cell Cycle. 2004 May;3(5):616-20. Epub 2004 May 15. Cell Cycle. 2004. PMID: 15044859 Review.
-
SV40 and cell cycle perturbations in malignant mesothelioma.Semin Cancer Biol. 2001 Feb;11(1):31-8. doi: 10.1006/scbi.2000.0344. Semin Cancer Biol. 2001. PMID: 11243897 Review.
Cited by
-
NF1 loss disrupts Schwann cell-axonal interactions: a novel role for semaphorin 4F.Genes Dev. 2008 Dec 1;22(23):3335-48. doi: 10.1101/gad.490608. Genes Dev. 2008. PMID: 19056885 Free PMC article.
-
HDAC3 Regulates the Transition to the Homeostatic Myelinating Schwann Cell State.Cell Rep. 2018 Dec 4;25(10):2755-2765.e5. doi: 10.1016/j.celrep.2018.11.045. Cell Rep. 2018. PMID: 30517863 Free PMC article.
-
Clinical correlation of molecular (VEGF, FGF, PDGF, c-Myc, c-Kit, Ras, p53) expression in juvenile nasopharyngeal angiofibroma.Eur Arch Otorhinolaryngol. 2018 Nov;275(11):2719-2726. doi: 10.1007/s00405-018-5110-5. Epub 2018 Aug 31. Eur Arch Otorhinolaryngol. 2018. PMID: 30171340
-
Association between melanocytic nevi and risk of breast diseases: The French E3N prospective cohort.PLoS Med. 2014 Jun 10;11(6):e1001660. doi: 10.1371/journal.pmed.1001660. eCollection 2014 Jun. PLoS Med. 2014. PMID: 24915306 Free PMC article.
-
The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation.EMBO J. 2004 Aug 4;23(15):3061-71. doi: 10.1038/sj.emboj.7600309. Epub 2004 Jul 8. EMBO J. 2004. PMID: 15241478 Free PMC article.
References
-
- Almoguera, C., D. Shibata, K. Forrester, J. Martin, N. Arnheim, and M. Perucho. 1988. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53:549-554. - PubMed
-
- Bartkova, J., J. Lukas, P. Guldberg, J. Alsner, A. F. Kirkin, J. Zeuthen, and J. Bartek. 1996. The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res. 56:5475-5483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
