The Ran GTPase cycle is required for yeast nuclear pore complex assembly
- PMID: 12654904
- PMCID: PMC2172763
- DOI: 10.1083/jcb.200209116
The Ran GTPase cycle is required for yeast nuclear pore complex assembly
Abstract
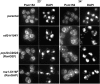
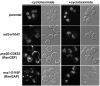
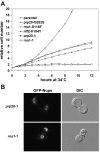
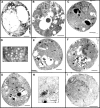
Here, we report the first evidence that the Ran GTPase cycle is required for nuclear pore complex (NPC) assembly. Using a genetic approach, factors required for NPC assembly were identified in Saccharomyces cerevisiae. Four mutant complementation groups were characterized that correspond to respective mutations in genes encoding Ran (gsp1), and essential Ran regulatory factors Ran GTPase-activating protein (rna1), Ran guanine nucleotide exchange factor (prp20), and the RanGDP import factor (ntf2). All the mutants showed temperature-dependent mislocalization of green fluorescence protein (GFP)-tagged nucleoporins (nups) and the pore-membrane protein Pom152. A decrease in GFP fluorescence associated with the nuclear envelope was observed along with an increase in the diffuse, cytoplasmic signal with GFP foci. The defects did not affect the stability of existing NPCs, and nup mislocalization was dependent on de novo protein synthesis and continued cell growth. Electron microscopy analysis revealed striking membrane perturbations and the accumulation of vesicles in arrested mutants. Using both biochemical fractionation and immunoelectron microscopy methods, these vesicles were shown to contain nups. We propose a model wherein a Ran-mediated vesicular fusion step is required for NPC assembly into intact nuclear envelopes.
Figures




Similar articles
-
The karyopherin Kap95 regulates nuclear pore complex assembly into intact nuclear envelopes in vivo.Mol Biol Cell. 2007 Mar;18(3):886-98. doi: 10.1091/mbc.e06-06-0525. Epub 2006 Dec 20. Mol Biol Cell. 2007. PMID: 17182855 Free PMC article.
-
Isolation and characterization of new Saccharomyces cerevisiae mutants perturbed in nuclear pore complex assembly.BMC Genet. 2002 Sep 5;3:17. doi: 10.1186/1471-2156-3-17. Epub 2002 Sep 5. BMC Genet. 2002. PMID: 12215173 Free PMC article.
-
The fission yeast Nup107-120 complex functionally interacts with the small GTPase Ran/Spi1 and is required for mRNA export, nuclear pore distribution, and proper cell division.Mol Cell Biol. 2004 Jul;24(14):6379-92. doi: 10.1128/MCB.24.14.6379-6392.2004. Mol Cell Biol. 2004. PMID: 15226438 Free PMC article.
-
Nuclear pores and nuclear assembly.Curr Opin Cell Biol. 2001 Jun;13(3):363-75. doi: 10.1016/s0955-0674(00)00221-0. Curr Opin Cell Biol. 2001. PMID: 11343909 Review.
-
Nuclear pores: sowing the seeds of assembly on the chromatin landscape.Curr Biol. 2003 Dec 16;13(24):R970-2. doi: 10.1016/j.cub.2003.11.046. Curr Biol. 2003. PMID: 14680657 Review. No abstract available.
Cited by
-
Defective nuclear import of Tpr in Progeria reflects the Ran sensitivity of large cargo transport.J Cell Biol. 2013 May 13;201(4):541-57. doi: 10.1083/jcb.201212117. Epub 2013 May 6. J Cell Biol. 2013. PMID: 23649804 Free PMC article.
-
Nuclear Pores Assemble from Nucleoporin Condensates During Oogenesis.Cell. 2019 Oct 17;179(3):671-686.e17. doi: 10.1016/j.cell.2019.09.022. Cell. 2019. PMID: 31626769 Free PMC article.
-
Border control at the nucleus: biogenesis and organization of the nuclear membrane and pore complexes.Dev Cell. 2009 Nov;17(5):606-16. doi: 10.1016/j.devcel.2009.10.007. Dev Cell. 2009. PMID: 19922866 Free PMC article. Review.
-
Recent advances in understanding nuclear size and shape.Nucleus. 2016 Apr 25;7(2):167-86. doi: 10.1080/19491034.2016.1162933. Epub 2016 Mar 10. Nucleus. 2016. PMID: 26963026 Free PMC article. Review.
-
Structure, Maintenance, and Regulation of Nuclear Pore Complexes: The Gatekeepers of the Eukaryotic Genome.Cold Spring Harb Perspect Biol. 2022 Mar 1;14(3):a040691. doi: 10.1101/cshperspect.a040691. Cold Spring Harb Perspect Biol. 2022. PMID: 34312247 Free PMC article. Review.
References
-
- Aitchison, J.D., M.P. Rout, M. Marelli, G. Blobel, and R.W. Wozniak. 1995. Two novel related yeast nucleoporins Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J. Cell Biol. 131:1133–1148. - PMC - PubMed
-
- Bamba, C., Y. Bobinnec, M. Fukuda, and E. Nishida. 2002. The GTPase Ran regulates chromosome positioning and nuclear envelope assembly in vivo. Curr. Biol. 12:503–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

