Dectin-1 mediates the biological effects of beta-glucans
- PMID: 12719478
- PMCID: PMC2193964
- DOI: 10.1084/jem.20021890
Dectin-1 mediates the biological effects of beta-glucans
Abstract
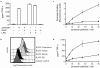
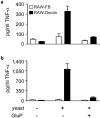
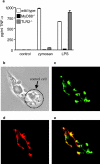
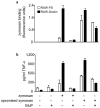
The ability of fungal-derived beta-glucan particles to induce leukocyte activation and the production of inflammatory mediators, such as tumor necrosis factor (TNF)-alpha, is a well characterized phenomenon. Although efforts have been made to understand how these carbohydrate polymers exert their immunomodulatory effects, the receptors involved in generating these responses are unknown. Here we show that Dectin-1 mediates the production of TNF-alpha in response to zymosan and live fungal pathogens, an activity that occurs at the cell surface and requires the cytoplasmic tail and immunoreceptor tyrosine activation motif of Dectin-1 as well as Toll-like receptor (TLR)-2 and Myd88. This is the first demonstration that the inflammatory response to pathogens requires recognition by a specific receptor in addition to the TLRs. Furthermore, these studies implicate Dectin-1 in the production of TNF-alpha in response to fungi, a critical step required for the successful control of these pathogens.
Figures
Similar articles
-
Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments.EMBO J. 2005 Mar 23;24(6):1277-86. doi: 10.1038/sj.emboj.7600594. Epub 2005 Feb 24. EMBO J. 2005. PMID: 15729357 Free PMC article.
-
Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2.J Exp Med. 2003 May 5;197(9):1107-17. doi: 10.1084/jem.20021787. Epub 2003 Apr 28. J Exp Med. 2003. PMID: 12719479 Free PMC article.
-
The role of SIGNR1 and the beta-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages.J Immunol. 2004 Jan 15;172(2):1157-62. doi: 10.4049/jimmunol.172.2.1157. J Immunol. 2004. PMID: 14707091
-
Dectin-1 and Dectin-2 in innate immunity against fungi.Int Immunol. 2011 Aug;23(8):467-72. doi: 10.1093/intimm/dxr046. Epub 2011 Jun 15. Int Immunol. 2011. PMID: 21677049 Review.
-
[Contribution of dectin-1 to the recognition of fungal cell wall products and the activation of innate immune response].Nihon Ishinkin Gakkai Zasshi. 2006;47(3):185-94. doi: 10.3314/jjmm.47.185. Nihon Ishinkin Gakkai Zasshi. 2006. PMID: 16940953 Review. Japanese.
Cited by
-
Immunostimulatory properties and antitumor activities of glucans (Review).Int J Oncol. 2013 Aug;43(2):357-64. doi: 10.3892/ijo.2013.1974. Epub 2013 Jun 5. Int J Oncol. 2013. PMID: 23739801 Free PMC article. Review.
-
Scavenger receptors and β-glucan receptors participate in the recognition of yeasts by murine macrophages.Inflamm Res. 2012 Feb;61(2):113-26. doi: 10.1007/s00011-011-0395-5. Epub 2011 Nov 25. Inflamm Res. 2012. PMID: 22116297 Free PMC article.
-
The mechanisms and cross-protection of trained innate immunity.Virol J. 2022 Dec 8;19(1):210. doi: 10.1186/s12985-022-01937-5. Virol J. 2022. PMID: 36482472 Free PMC article. Review.
-
Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments.EMBO J. 2005 Mar 23;24(6):1277-86. doi: 10.1038/sj.emboj.7600594. Epub 2005 Feb 24. EMBO J. 2005. PMID: 15729357 Free PMC article.
-
TLR2 modulates inflammation in zymosan-induced arthritis in mice.Arthritis Res Ther. 2005;7(2):R370-9. doi: 10.1186/ar1494. Epub 2005 Jan 21. Arthritis Res Ther. 2005. PMID: 15743485 Free PMC article.
References
-
- Czop, J.K. 1986. The role of beta-glucan receptors on blood and tissue leukocytes in phagocytosis and metabolic activation. Pathol. Immunopathol. Res. 5:286–296. - PubMed
-
- Ross, G.D., V. Vetvicka, J. Yan, Y. Xia, and J. Vetvickova. 1999. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 42:61–74. - PubMed
-
- Williams, D.L., A. Mueller, and W. Browder. 1996. Glucan-based macrophage stimulators. Clinical Immunotherapy. 5:392–399.
-
- Riggi, S.J., and N.R. Di Luzio. 1961. Identification of a reticuloendothelial stimulating agent in zymosan. Am. J. Physiol. 200:297–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

