Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis
- PMID: 12796513
- PMCID: PMC164656
- DOI: 10.1073/pnas.1232420100
Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis
Abstract
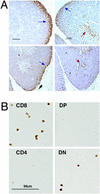
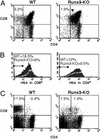
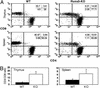
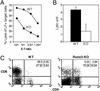
The RUNX transcription factors are important regulators of lineage-specific gene expression. RUNX are bifunctional, acting both as activators and repressors of tissue-specific _target genes. Recently, we have demonstrated that Runx3 is a neurogenic transcription factor, which regulates development and survival of proprioceptive neurons in dorsal root ganglia. Here we report that Runx3 and Runx1 are highly expressed in thymic medulla and cortex, respectively, and function in development of CD8 T cells during thymopoiesis. Runx3-deficient (Runx3 KO) mice display abnormalities in CD4 expression during lineage decisions and impairment of CD8 T cell maturation in the thymus. A large proportion of Runx3 KO peripheral CD8 T cells also expressed CD4, and in contrast to wild-type, their proliferation ability was largely reduced. In addition, the in vitro cytotoxic activity of alloimmunized peritoneal exudate lymphocytes was significantly lower in Runx3 KO compared with WT mice. In a compound mutant mouse, null for Runx3 and heterozygous for Runx1 (Runx3-/-;Runx1+/-), all peripheral CD8 T cells also expressed CD4, resulting in a complete lack of single-positive CD8+ T cells in the spleen. The results provide information on the role of Runx3 and Runx1 in thymopoiesis and suggest that both act as transcriptional repressors of CD4 expression during T cell lineage decisions.
Figures


Similar articles
-
The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells.J Exp Med. 2007 Aug 6;204(8):1945-57. doi: 10.1084/jem.20070133. Epub 2007 Jul 23. J Exp Med. 2007. PMID: 17646406 Free PMC article.
-
Runx3 regulates integrin alpha E/CD103 and CD4 expression during development of CD4-/CD8+ T cells.J Immunol. 2005 Aug 1;175(3):1694-705. doi: 10.4049/jimmunol.175.3.1694. J Immunol. 2005. PMID: 16034110
-
Regulation of Th-POK and Runx3 in T cell development in human thymoma.Autoimmunity. 2009;42(8):653-60. doi: 10.3109/08916930903120941. Autoimmunity. 2009. PMID: 19886737
-
Runx and ThPOK: a balancing act to regulate thymocyte lineage commitment.J Cell Biochem. 2009 Aug 15;107(6):1037-45. doi: 10.1002/jcb.22212. J Cell Biochem. 2009. PMID: 19479890 Review.
-
The CD4/CD8 lineages: central decisions and peripheral modifications for T lymphocytes.Curr Top Microbiol Immunol. 2014;373:113-29. doi: 10.1007/82_2013_323. Curr Top Microbiol Immunol. 2014. PMID: 23612990 Review.
Cited by
-
Goldmine integrates information placing genomic ranges into meaningful biological contexts.Nucleic Acids Res. 2016 Jul 8;44(12):5550-6. doi: 10.1093/nar/gkw477. Epub 2016 Jun 1. Nucleic Acids Res. 2016. PMID: 27257071 Free PMC article.
-
Runx2 transcriptome of prostate cancer cells: insights into invasiveness and bone metastasis.Mol Cancer. 2010 Sep 23;9:258. doi: 10.1186/1476-4598-9-258. Mol Cancer. 2010. PMID: 20863401 Free PMC article.
-
Runx3-mediated transcriptional program in cytotoxic lymphocytes.PLoS One. 2013 Nov 13;8(11):e80467. doi: 10.1371/journal.pone.0080467. eCollection 2013. PLoS One. 2013. PMID: 24236182 Free PMC article.
-
PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation.J Cell Sci. 2009 May 1;122(Pt 9):1382-9. doi: 10.1242/jcs.040709. Epub 2009 Apr 7. J Cell Sci. 2009. PMID: 19351720 Free PMC article.
-
Runx2 is required for activity of CD44+/CD24-/low breast cancer stem cell in breast cancer development.Am J Transl Res. 2020 May 15;12(5):2305-2318. eCollection 2020. Am J Transl Res. 2020. PMID: 32509221 Free PMC article.
References
-
- Levanon, D., Negreanu, V., Bernstein, Y., Bar-Am, I., Avivi, L. & Groner, Y. (1994) Genomics 23, 425-432. - PubMed
-
- Avraham, K. B., Levanon, D., Negreanu, V., Bernstein, Y., Groner, Y., Copeland, N. G. & Jenkins, N. A. (1995) Genomics 25, 603-605. - PubMed
-
- Bae, S. C., Takahashi, E., Zhang, Y. W., Ogawa, E., Shigesada, K., Namba, Y., Satake, M. & Ito, Y. (1995) Gene 159, 245-248. - PubMed
-
- Calabi, F., Rhodes, M., Williamson, P. & Boyd, Y. (1995) Genomics 26, 607-610. - PubMed
-
- Tracey, W. D. & Speck, N. A. (2000) Semin. Cell. Dev. Biol. 11, 337-342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

