Intracellular patch electrochemistry: regulation of cytosolic catecholamines in chromaffin cells
- PMID: 12843288
- PMCID: PMC6741260
- DOI: 10.1523/JNEUROSCI.23-13-05835.2003
Intracellular patch electrochemistry: regulation of cytosolic catecholamines in chromaffin cells
Abstract
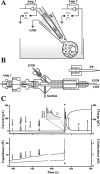
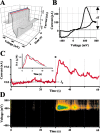
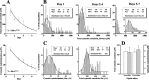
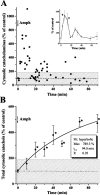
Alterations in the cytosolic pool directly affect neurotransmitter synthesis and release and are suggested to be key factors in various neurodegenerative disorders. Although this cytosolic pool is the most metabolically active, it is miniscule compared with the amount of vesicular transmitter and has never been quantified separately. Here, we introduce intracellular patch electrochemistry (IPE), a technique that for the first time provides direct measurements of cytosolic oxidizable molecules in single mammalian cells. In amperometric mode, IPE detects total catechols, whereas in cyclic voltammetric mode, it preferentially measures catecholamines. In cultured chromaffin cells, the total cytosolic catechol concentration was 50-500 microm, of which approximately 10% were catecholamines. Reserpine, a vesicular monoamine transporter inhibitor, had no effect on the catecholamine pool but increased total catechols by fourfold to fivefold. Combined with pargyline, a monoamine oxidase inhibitor, reserpine increased catecholamine levels in the cytosol by approximately sixfold. Amphetamine induced a transient approximately fivefold accumulation of cytosolic catecholamines and a slow increase of total catechols. In cells incubated with 3,4-dihydroxy-L-phenylalanine (L-DOPA), catecholamines increased by approximately 2.5-fold and total catechols increased by approximately fourfold. Cytosolic catecholamines returned to control levels <or=10 min after L-DOPA withdrawal, whereas total catechols remained approximately twofold elevated even after a 1.5 hr incubation in L-DOPA-free media. Our data indicate that cytosolic catecholamines are strictly maintained at a defined level, and drug-induced increases in their concentrations lead to the accumulation of other catecholamine derivatives, such as DOPAC and 3,4-dihydroxyphenylethyleneglycol. These derivatives reside in the cytosol for hours after treatment and may be an underlying cause of drug-related cytotoxicity.
Figures


Similar articles
-
Patch Amperometry and Intracellular Patch Electrochemistry.Methods Mol Biol. 2023;2565:239-260. doi: 10.1007/978-1-0716-2671-9_17. Methods Mol Biol. 2023. PMID: 36205899 Free PMC article.
-
Alpha-synuclein overexpression increases cytosolic catecholamine concentration.J Neurosci. 2006 Sep 6;26(36):9304-11. doi: 10.1523/JNEUROSCI.0519-06.2006. J Neurosci. 2006. PMID: 16957086 Free PMC article.
-
Chromogranin B gene ablation reduces the catecholamine cargo and decelerates exocytosis in chromaffin secretory vesicles.J Neurosci. 2010 Jan 20;30(3):950-7. doi: 10.1523/JNEUROSCI.2894-09.2010. J Neurosci. 2010. PMID: 20089903 Free PMC article.
-
Roles of catechol neurochemistry in autonomic function testing.Clin Auton Res. 2018 Jun;28(3):273-288. doi: 10.1007/s10286-018-0528-9. Epub 2018 Apr 28. Clin Auton Res. 2018. PMID: 29705971 Free PMC article. Review.
-
Mode of action of psychomotor stimulant drugs.Int Rev Neurobiol. 1970;12:307-83. doi: 10.1016/s0074-7742(08)60065-3. Int Rev Neurobiol. 1970. PMID: 4918147 Review. No abstract available.
Cited by
-
Increased Vesicular Monoamine Transporter 2 (VMAT2; Slc18a2) Protects against Methamphetamine Toxicity.ACS Chem Neurosci. 2015 May 20;6(5):790-9. doi: 10.1021/acschemneuro.5b00010. Epub 2015 Mar 9. ACS Chem Neurosci. 2015. PMID: 25746685 Free PMC article.
-
Amphetamine induces dopamine efflux through a dopamine transporter channel.Proc Natl Acad Sci U S A. 2005 Mar 1;102(9):3495-500. doi: 10.1073/pnas.0407737102. Epub 2005 Feb 22. Proc Natl Acad Sci U S A. 2005. PMID: 15728379 Free PMC article.
-
N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux.PLoS Biol. 2004 Mar;2(3):E78. doi: 10.1371/journal.pbio.0020078. Epub 2004 Mar 16. PLoS Biol. 2004. PMID: 15024426 Free PMC article.
-
A specific survival response in dopamine neurons at most risk in Parkinson's disease.J Neurosci. 2006 Sep 20;26(38):9750-60. doi: 10.1523/JNEUROSCI.2745-06.2006. J Neurosci. 2006. PMID: 16988046 Free PMC article.
-
Live imaging of synaptic vesicle release and retrieval in dopaminergic neurons.Front Neural Circuits. 2009 Jun 1;3:3. doi: 10.3389/neuro.04.003.2009. eCollection 2009. Front Neural Circuits. 2009. PMID: 19521540 Free PMC article.
References
-
- Ahlskog JE, Uitti RJ, Low PA, Tyce GM, O'Brien JF, Nickander KK ( 1996) Levodopa and deprenyl treatment effects on peripheral indices of oxidant stress in Parkinson's disease. Neurology 46: 796-801. - PubMed
-
- Albillos A, Dernick G, Hostmann H, Almers W, Alvarez de Toledo G, Lindau M ( 1997) The exocytic event in chromaffin cells revealed by patch amperometry. Nature 389: 509-512. - PubMed
-
- Anderson BB, Chen G, Gutman DA, Ewing AG ( 1998) Dopamine levels of two classes of vesicles are differentially depleted by amphetamine. Brain Res 788: 294-301. - PubMed
-
- Bader MF, Holz RW, Kumakura K, Vitale N ( 2002) Exocytosis: the chromaffin cell as a model system. Ann NY Acad Sci 971: 178-183. - PubMed
-
- Baur JE, Kristensen EW, May LJ, Wiedemann DJ, Wightman RM ( 1988) Fast-scan voltammetry of biogenic amines. Anal Chem 60: 1268-1272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
