Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis
- PMID: 12847083
- PMCID: PMC2172724
- DOI: 10.1083/jcb.200302084
Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis
Abstract
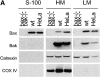
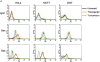
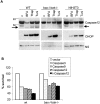
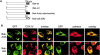
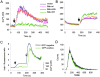
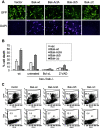
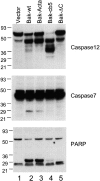
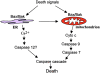
Bax and Bak play a redundant but essential role in apoptosis initiated by the mitochondrial release of apoptogenic factors. In addition to their presence at the mitochondrial outer membrane, Bax and Bak can also localize to the ER. Agents that initiate ER stress responses can induce conformational changes and oligomerization of Bax on the ER as well as on mitochondria. In wild-type cells, this is associated with caspase 12 cleavage that is abolished in bax-/-bak-/- cells. In bax-/-bak-/- cells, introduction of Bak mutants selectively _targeted to either mitochondria or the ER can induce apoptosis. However, ER-_targeted, but not mitochondria-_targeted, Bak leads to progressive depletion of ER Ca2+ and induces caspase 12 cleavage. In contrast, mitochondria-_targeted Bak leads to enhanced caspase 7 and PARP cleavage in comparison with the ER-_targeted Bak. These findings demonstrate that in addition to their functions at mitochondria, Bax and Bak also localize to the ER and function to initiate a parallel pathway of caspase activation and apoptosis.
Figures




Similar articles
-
BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis.Science. 2003 Apr 4;300(5616):135-9. doi: 10.1126/science.1081208. Epub 2003 Mar 6. Science. 2003. PMID: 12624178
-
Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release.Cancer Res. 2003 Apr 1;63(7):1712-21. Cancer Res. 2003. PMID: 12670926
-
Inhibition of the ER Ca2+ pump forces multidrug-resistant cells deficient in Bak and Bax into necrosis.J Cell Sci. 2009 Dec 15;122(Pt 24):4481-91. doi: 10.1242/jcs.055772. Epub 2009 Nov 17. J Cell Sci. 2009. PMID: 19920074
-
Regulation of endoplasmic reticulum Ca2+ dynamics by proapoptotic BCL-2 family members.Biochem Pharmacol. 2003 Oct 15;66(8):1335-40. doi: 10.1016/s0006-2952(03)00482-9. Biochem Pharmacol. 2003. PMID: 14555206 Review.
-
New insights in the role of Bcl-2 Bcl-2 and the endoplasmic reticulum.Apoptosis. 2002 Oct;7(5):441-7. doi: 10.1023/a:1020087108926. Apoptosis. 2002. PMID: 12207177 Review.
Cited by
-
Cell intrinsic and extrinsic activators of the unfolded protein response in cancer: Mechanisms and _targets for therapy.Semin Cancer Biol. 2015 Aug;33:3-15. doi: 10.1016/j.semcancer.2015.04.002. Epub 2015 Apr 25. Semin Cancer Biol. 2015. PMID: 25920797 Free PMC article. Review.
-
Klotho alleviates chronic intermittent hypoxia-induced genioglossus myocyte apoptosis by inhibiting endoplasmic reticulum stress.Exp Ther Med. 2021 Jul;22(1):708. doi: 10.3892/etm.2021.10140. Epub 2021 May 2. Exp Ther Med. 2021. PMID: 34007317 Free PMC article.
-
Regulatory Role of Sphingosine-1-Phosphate and C16:0 Ceramide, in Immunogenic Cell Death of Colon Cancer Cells Induced by Bak/Bax-Activation.Cancers (Basel). 2022 Oct 22;14(21):5182. doi: 10.3390/cancers14215182. Cancers (Basel). 2022. PMID: 36358599 Free PMC article.
-
Differential contribution of Puma and Noxa in dual regulation of p53-mediated apoptotic pathways.EMBO J. 2006 Oct 18;25(20):4952-62. doi: 10.1038/sj.emboj.7601359. Epub 2006 Oct 5. EMBO J. 2006. PMID: 17024184 Free PMC article.
-
A mechanism of ineffective erythropoiesis in β-thalassemia/Hb E disease.Haematologica. 2010 May;95(5):716-23. doi: 10.3324/haematol.2009.015701. Epub 2009 Dec 16. Haematologica. 2010. PMID: 20015891 Free PMC article.
References
-
- Abeele, F.V., R. Skryma, Y. Shuba, F. Van Coppenolle, C. Slomianny, M. Roudbaraki, B. Mauroy, F. Wuytack, and N. Prevarskaya. 2002. Bcl-2-dependent modulation of Ca(2+) homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 1:169–179. - PubMed
-
- Akao, Y., Y. Otsuki, S. Kataoka, Y. Ito, and Y. Tsujimoto. 1994. Multiple subcellular localization of bcl-2: detection in nuclear outer membrane, endoplasmic reticulum membrane, and mitochondrial membranes. Cancer Res. 54:2468–2471. - PubMed
-
- Annis, M.G., N. Zamzami, W. Zhu, L.Z. Penn, G. Kroemer, B. Leber, and D.W. Andrews. 2001. Endoplasmic reticulum localized Bcl-2 prevents apoptosis when redistribution of cytochrome c is a late event. Oncogene. 20:1939–1952. - PubMed
-
- Cecconi, F., G. Alvarez-Bolado, B.I. Meyer, K.A. Roth, and P. Gruss. 1998. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 94:727–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

