Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia
- PMID: 12867516
- PMCID: PMC6740534
- DOI: 10.1523/JNEUROSCI.23-15-06315.2003
Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia
Abstract
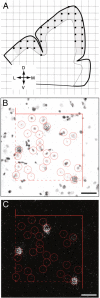
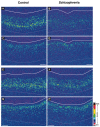
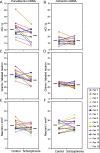
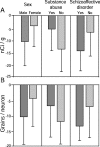
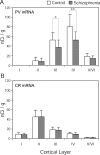
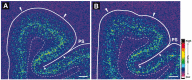
Markers of inhibitory neurotransmission are altered in the prefrontal cortex (PFC) of subjects with schizophrenia, and several lines of evidence suggest that these alterations may be most prominent in the subset of GABA-containing neurons that express the calcium-binding protein, parvalbumin (PV). To test this hypothesis, we evaluated the expression of mRNAs for PV, another calcium-binding protein, calretinin (CR), and glutamic acid decarboxylase (GAD67) in postmortem brain specimens from 15 pairs of subjects with schizophrenia and matched control subjects using single- and dual-label in situ hybridization. Signal intensity for PV mRNA expression in PFC area 9 was significantly decreased in the subjects with schizophrenia, predominantly in layers III and IV. Analysis at the cellular level revealed that this decrease was attributable principally to a reduction in PV mRNA expression per neuron rather than by a decreased density of PV mRNA-positive neurons. In contrast, the same measures of CR mRNA expression were not altered in schizophrenia. These findings were confirmed by findings from cDNA microarray studies using different probes. Across the subjects with schizophrenia, the decrease in neuronal PV mRNA expression was highly associated (r = 0.84) with the decrease in the density of neurons containing detectable levels of GAD67 mRNA. Furthermore, simultaneous detection of PV and GAD67 mRNAs revealed that in subjects with schizophrenia only 55% of PV mRNA-positive neurons had detectable levels of GAD67 mRNA. Given the critical role that PV-containing GABA neurons appear to play in regulating the cognitive functions mediated by the PFC, the selective alterations in gene expression in these neurons may contribute to the cognitive deficits characteristic of schizophrenia.
Figures



Similar articles
-
Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia.Arch Gen Psychiatry. 2000 Mar;57(3):237-45. doi: 10.1001/archpsyc.57.3.237. Arch Gen Psychiatry. 2000. PMID: 10711910
-
Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia.J Neurosci. 2005 Jan 12;25(2):372-83. doi: 10.1523/JNEUROSCI.4035-04.2005. J Neurosci. 2005. PMID: 15647480 Free PMC article.
-
GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons.Am J Psychiatry. 2001 Feb;158(2):256-65. doi: 10.1176/appi.ajp.158.2.256. Am J Psychiatry. 2001. PMID: 11156808
-
[Schizophrenia and cortical GABA neurotransmission].Seishin Shinkeigaku Zasshi. 2010;112(5):439-52. Seishin Shinkeigaku Zasshi. 2010. PMID: 20560363 Review. Japanese.
-
Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction.Physiol Behav. 2002 Dec;77(4-5):501-5. doi: 10.1016/s0031-9384(02)00936-8. Physiol Behav. 2002. PMID: 12526990 Review.
Cited by
-
The Role of Parvalbumin Interneuron GIRK Signaling in the Regulation of Affect and Cognition in Male and Female Mice.Front Behav Neurosci. 2021 Mar 26;15:621751. doi: 10.3389/fnbeh.2021.621751. eCollection 2021. Front Behav Neurosci. 2021. PMID: 33841107 Free PMC article.
-
BDNF rescues prefrontal dysfunction elicited by pyramidal neuron-specific DTNBP1 deletion in vivo.J Mol Cell Biol. 2017 Apr 1;9(2):117-131. doi: 10.1093/jmcb/mjw029. J Mol Cell Biol. 2017. PMID: 27330059 Free PMC article.
-
Circuit- and Diagnosis-Specific DNA Methylation Changes at γ-Aminobutyric Acid-Related Genes in Postmortem Human Hippocampus in Schizophrenia and Bipolar Disorder.JAMA Psychiatry. 2015 Jun;72(6):541-51. doi: 10.1001/jamapsychiatry.2015.49. JAMA Psychiatry. 2015. PMID: 25738424 Free PMC article.
-
NMDA antagonists recreate signal-to-noise ratio and timing perturbations present in schizophrenia.Neurobiol Dis. 2012 Apr;46(1):93-100. doi: 10.1016/j.nbd.2011.12.049. Epub 2012 Jan 9. Neurobiol Dis. 2012. PMID: 22245663 Free PMC article.
-
Interneuron dysfunction in psychiatric disorders.Nat Rev Neurosci. 2012 Jan 18;13(2):107-20. doi: 10.1038/nrn3155. Nat Rev Neurosci. 2012. PMID: 22251963 Review.
References
-
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney Jr WE, Jones EG ( 1995) Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 52: 258–266. - PubMed
-
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Lewis DA ( 1999) Lamina-specific alteration in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 156: 1580–1589. - PubMed
-
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K ( 1996) Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun 229: 891–895. - PubMed
-
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP ( 2002) Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry 52: 708–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
