Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents
- PMID: 12930805
- PMCID: PMC6740746
- DOI: 10.1523/JNEUROSCI.23-20-07659.2003
Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents
Abstract
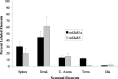
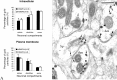
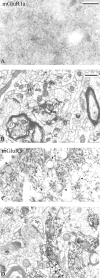
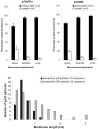
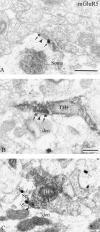
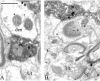
Group I metabotropic glutamate receptors (mGluRs) are involved in long-term synaptic plasticity and neuroprotection in the striatum, but the specific role(s) of mGluR1 and mGluR5 remain poorly understood. In this study, we used electron-microscopic immunocytochemistry to compare the pattern of subsynaptic and subcellular distribution of mGluR1a and mGluR5 in relation to putative glutamatergic and dopaminergic inputs to the monkey striatum. At the light-microscopic level, both group I mGluRs are expressed in the striatal neuropil. In addition, numerous perikarya of striatal output neurons are immunostained for mGluR5, but much less frequently for mGluR1a. At the electron-microscopic level, immunoreactivity for both receptor subtypes is primarily expressed postsynaptically in dendrites and spines, although presynaptic mGluR1a labeling of glutamatergic thalamostriatal boutons and, less frequently, dopaminergic and corticostriatal terminals is also seen. In contrast to mGluR1a, mGluR5 immunoreactivity is rarely encountered presynaptically. In postsynaptic elements, 40-70% of immunoreactivity for both receptor subtypes is expressed intracellularly, whereas 30-60% is apposed to the plasma membrane. More than 80% of the labeling apposed to the plasma membrane is extrasynaptic. The remaining 20% is located at the edges of putative glutamatergic synapses or in the active zone of symmetric synapses. In mGluR5-, but not mGluR1a-immunostained sections, approximately 70% of dopaminergic symmetric synapses are labeled perisynaptically. These data emphasize the differential pattern of subsynaptic localization of the two group I mGluRs and provide various presynaptic and postsynaptic sites whereby mGluR1 and mGluR5 could mediate different, but complementary, effects on glutamatergic and dopaminergic transmission in the primate striatum.
Figures


Similar articles
-
Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus.J Comp Neurol. 2004 Jul 5;474(4):589-602. doi: 10.1002/cne.20158. J Comp Neurol. 2004. PMID: 15174075
-
Group I metabotropic glutamate receptors in the primate motor thalamus: subsynaptic association with cortical and sub-cortical glutamatergic afferents.Brain Struct Funct. 2019 Nov;224(8):2787-2804. doi: 10.1007/s00429-019-01937-2. Epub 2019 Aug 17. Brain Struct Funct. 2019. PMID: 31422483
-
Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra.J Neurosci. 2001 Mar 15;21(6):1838-47. doi: 10.1523/JNEUROSCI.21-06-01838.2001. J Neurosci. 2001. PMID: 11245668 Free PMC article.
-
GABA(B) and group I metabotropic glutamate receptors in the striatopallidal complex in primates.J Anat. 2000 May;196 ( Pt 4)(Pt 4):555-76. doi: 10.1046/j.1469-7580.2000.19640555.x. J Anat. 2000. PMID: 10923987 Free PMC article. Review.
-
Postsynaptic integration of glutamatergic and dopaminergic signals in the striatum.Prog Neurobiol. 1994 Oct;44(2):163-96. doi: 10.1016/0301-0082(94)90037-x. Prog Neurobiol. 1994. PMID: 7831476 Review.
Cited by
-
Inositol 1,4,5-triphosphate drives glutamatergic and cholinergic inhibition selectively in spiny projection neurons in the striatum.J Neurosci. 2013 Feb 6;33(6):2697-708. doi: 10.1523/JNEUROSCI.4759-12.2013. J Neurosci. 2013. PMID: 23392696 Free PMC article.
-
P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2.Proc Natl Acad Sci U S A. 2005 May 31;102(22):8042-7. doi: 10.1073/pnas.0408818102. Epub 2005 May 18. Proc Natl Acad Sci U S A. 2005. PMID: 15901899 Free PMC article.
-
Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum.J Neurosci. 2007 Apr 4;27(14):3663-76. doi: 10.1523/JNEUROSCI.0448-07.2007. J Neurosci. 2007. PMID: 17409230 Free PMC article.
-
SEQUIN Multiscale Imaging of Mammalian Central Synapses Reveals Loss of Synaptic Connectivity Resulting from Diffuse Traumatic Brain Injury.Neuron. 2020 Jul 22;107(2):257-273.e5. doi: 10.1016/j.neuron.2020.04.012. Epub 2020 May 8. Neuron. 2020. PMID: 32392471 Free PMC article.
-
Control of striatal signaling by g protein regulators.Front Neuroanat. 2011 Aug 8;5:49. doi: 10.3389/fnana.2011.00049. eCollection 2011. Front Neuroanat. 2011. PMID: 21852966 Free PMC article.
References
-
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S ( 1992) Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca +2 signal transduction. J Biol Chem 267: 13361-13368. - PubMed
-
- Awad-Greko H, Conn PJ ( 2001) Activation of groups I and III metabotropic glutamate receptors inhibits excitatory transmission in the rat subthalamic nucleus. Neuropharmacology 41: 32-41. - PubMed
-
- Battaglia G, Bruno V, Pisani A, Centonze D, Catania MV, Calabresi P, Nicoletti F ( 2001) Selective blockade of type-1 metabotropic glutamate receptors induces neuroprotection by enhancing GABAergic transmission. Mol Cell Neurosci 17: 1071-1083. - PubMed
-
- Bradley SD, Standaert DG, Rhodes KJ, Reese HD, Testa CM, Levey AI, Conn PJ ( 1999) Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol 407: 33-46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
