Pten dose dictates cancer progression in the prostate
- PMID: 14691534
- PMCID: PMC270016
- DOI: 10.1371/journal.pbio.0000059
Pten dose dictates cancer progression in the prostate
Abstract
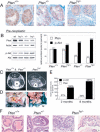
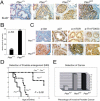
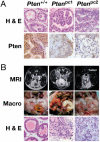
Complete inactivation of the PTEN tumor suppressor gene is extremely common in advanced cancer, including prostate cancer (CaP). However, one PTEN allele is already lost in the vast majority of CaPs at presentation. To determine the consequence of PTEN dose variations on cancer progression, we have generated by homologous recombination a hypomorphic Pten mouse mutant series with decreasing Pten activity: Pten(hy/+) > Pten(+/-) > Pten(hy/-) (mutants in which we have rescued the embryonic lethality due to complete Pten inactivation) > Pten prostate conditional knockout (Pten(pc)) mutants. In addition, we have generated and comparatively analyzed two distinct Pten(pc) mutants in which Pten is inactivated focally or throughout the entire prostatic epithelium. We find that the extent of Pten inactivation dictate in an exquisite dose-dependent fashion CaP progression, its incidence, latency, and biology. The dose of Pten affects key downstream _targets such as Akt, p27(Kip1), mTOR, and FOXO3. Our results provide conclusive genetic support for the notion that PTEN is haploinsufficient in tumor suppression and that its dose is a key determinant in cancer progression.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures



Similar articles
-
PTEN deficiency is fully penetrant for prostate adenocarcinoma in C57BL/6 mice via mTOR-dependent growth.Am J Pathol. 2009 May;174(5):1869-79. doi: 10.2353/ajpath.2009.080055. Am J Pathol. 2009. PMID: 19395652 Free PMC article.
-
Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression.J Biol Chem. 2000 Aug 11;275(32):24500-5. doi: 10.1074/jbc.M003145200. J Biol Chem. 2000. PMID: 10827191
-
Cooperation between FGF8b overexpression and PTEN deficiency in prostate tumorigenesis.Cancer Res. 2006 Feb 15;66(4):2188-94. doi: 10.1158/0008-5472.CAN-05-3440. Cancer Res. 2006. PMID: 16489020
-
Akt-regulated pathways in prostate cancer.Oncogene. 2005 Nov 14;24(50):7465-74. doi: 10.1038/sj.onc.1209096. Oncogene. 2005. PMID: 16288293 Review.
-
The Par-4/PTEN connection in tumor suppression.Cell Cycle. 2009 Aug 15;8(16):2518-22. doi: 10.4161/cc.8.16.9384. Epub 2009 Aug 29. Cell Cycle. 2009. PMID: 19625770 Free PMC article. Review.
Cited by
-
Functional screen analysis reveals miR-26b and miR-128 as central regulators of pituitary somatomammotrophic tumor growth through activation of the PTEN-AKT pathway.Oncogene. 2013 Mar 28;32(13):1651-9. doi: 10.1038/onc.2012.190. Epub 2012 May 21. Oncogene. 2013. PMID: 22614013 Free PMC article.
-
Disruption of Abi1/Hssh3bp1 expression induces prostatic intraepithelial neoplasia in the conditional Abi1/Hssh3bp1 KO mice.Oncogenesis. 2012 Sep 3;1(9):e26. doi: 10.1038/oncsis.2012.28. Oncogenesis. 2012. PMID: 23552839 Free PMC article.
-
Systemic elevation of PTEN induces a tumor-suppressive metabolic state.Cell. 2012 Mar 30;149(1):49-62. doi: 10.1016/j.cell.2012.02.030. Epub 2012 Mar 6. Cell. 2012. PMID: 22401813 Free PMC article.
-
SPLUNC1 regulates cell progression and apoptosis through the miR-141-PTEN/p27 pathway, but is hindered by LMP1.PLoS One. 2013;8(3):e56929. doi: 10.1371/journal.pone.0056929. Epub 2013 Mar 5. PLoS One. 2013. PMID: 23472073 Free PMC article.
-
Molecular circuit involving KLK4 integrates androgen and mTOR signaling in prostate cancer.Proc Natl Acad Sci U S A. 2013 Jul 9;110(28):E2572-81. doi: 10.1073/pnas.1304318110. Epub 2013 Jun 24. Proc Natl Acad Sci U S A. 2013. PMID: 23798432 Free PMC article.
References
-
- Abate-Shen C, Banach-Petrosky WA, Sun X, Economides KD, Desai N, et al. Nkx3.1: Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res. 2003;63:3886–3890. - PubMed
-
- Backman S, Stambolic V, Mak T. PTEN function in mammalian cell size regulation. Curr Opin Neurobiol. 2002;12:516–522. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
-
- Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, et al. Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst. 1998;90:1284–1291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

