Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model
- PMID: 15339646
- PMCID: PMC2442162
- DOI: 10.1016/j.neuron.2004.08.013
Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model
Abstract
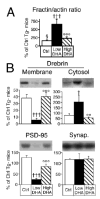
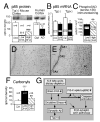
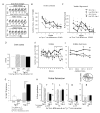
Learning and memory depend on dendritic spine actin assembly and docosahexaenoic acid (DHA), an essential n-3 (omega-3) polyunsaturated fatty acid (PFA). High DHA consumption is associated with reduced Alzheimer's disease (AD) risk, yet mechanisms and therapeutic potential remain elusive. Here, we report that reduction of dietary n-3 PFA in an AD mouse model resulted in 80%-90% losses of the p85alpha subunit of phosphatidylinositol 3-kinase and the postsynaptic actin-regulating protein drebrin, as in AD brain. The loss of postsynaptic proteins was associated with increased oxidation, without concomitant neuron or presynaptic protein loss. n-3 PFA depletion increased caspase-cleaved actin, which was localized in dendrites ultrastructurally. Treatment of n-3 PFA-restricted mice with DHA protected against these effects and behavioral deficits and increased antiapoptotic BAD phosphorylation. Since n-3 PFAs are essential for p85-mediated CNS insulin signaling and selective protection of postsynaptic proteins, these findings have implications for neurodegenerative diseases where synaptic loss is critical, especially AD.
Figures



Comment in
-
Food for thought: essential fatty acid protects against neuronal deficits in transgenic mouse model of AD.Neuron. 2004 Sep 2;43(5):596-9. doi: 10.1016/j.neuron.2004.08.025. Neuron. 2004. PMID: 15339638 Review.
Similar articles
-
Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease.Eur J Neurosci. 2005 Aug;22(3):617-26. doi: 10.1111/j.1460-9568.2005.04253.x. Eur J Neurosci. 2005. PMID: 16101743
-
Food for thought: essential fatty acid protects against neuronal deficits in transgenic mouse model of AD.Neuron. 2004 Sep 2;43(5):596-9. doi: 10.1016/j.neuron.2004.08.025. Neuron. 2004. PMID: 15339638 Review.
-
Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer's disease mouse model.Nutr Health. 2006;18(3):249-59. doi: 10.1177/026010600601800307. Nutr Health. 2006. PMID: 17180870 Review.
-
Making of a Synapse: Recurrent Roles of Drebrin A at Excitatory Synapses Throughout Life.Adv Exp Med Biol. 2017;1006:119-139. doi: 10.1007/978-4-431-56550-5_8. Adv Exp Med Biol. 2017. PMID: 28865018 Review.
-
Drebrin, a dendritic spine protein, is manifold decreased in brains of patients with Alzheimer's disease and Down syndrome.Neurosci Lett. 2002 May 24;324(3):209-12. doi: 10.1016/s0304-3940(02)00210-0. Neurosci Lett. 2002. PMID: 12009525
Cited by
-
A Novel Combination of Docosahexaenoic Acid, All-Trans Retinoic Acid, and 1, 25-Dihydroxyvitamin D3 Reduces T-Bet Gene Expression, Serum Interferon Gamma, and Clinical Scores but Promotes PPARγ Gene Expression in Experimental Autoimmune Encephalomyelitis.J Mol Neurosci. 2016 Dec;60(4):498-508. doi: 10.1007/s12031-016-0834-4. Epub 2016 Sep 19. J Mol Neurosci. 2016. PMID: 27647308
-
Cognitive reserve, cortical plasticity and resistance to Alzheimer's disease.Alzheimers Res Ther. 2012 Mar 1;4(2):7. doi: 10.1186/alzrt105. Alzheimers Res Ther. 2012. PMID: 22380508 Free PMC article.
-
Oral supplementation with docosahexaenoic acid and uridine-5'-monophosphate increases dendritic spine density in adult gerbil hippocampus.Brain Res. 2007 Nov 28;1182:50-9. doi: 10.1016/j.brainres.2007.08.089. Epub 2007 Sep 21. Brain Res. 2007. PMID: 17950710 Free PMC article.
-
The Decrease of n-3 Fatty Acid Energy Percentage in an Equicaloric Diet Fed to B6C3Fe Mice for Three Generations Elicits Obesity.Cardiovasc Psychiatry Neurol. 2009;2009:867041. doi: 10.1155/2009/867041. Epub 2009 Sep 16. Cardiovasc Psychiatry Neurol. 2009. PMID: 20029635 Free PMC article.
-
DHA may prevent age-related dementia.J Nutr. 2010 Apr;140(4):869-74. doi: 10.3945/jn.109.113910. Epub 2010 Feb 24. J Nutr. 2010. PMID: 20181786 Free PMC article. Review.
References
-
- Akbar M, Kim HY. Protective effects of docosahexaenoic acid in staurosporine-induced apoptosis: involvement of phosphatidylinositol-3 kinase pathway. J Neurochem. 2002;82:655–665. - PubMed
-
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience. 2001;103:373–383. - PubMed
-
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. - PubMed
-
- Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG10685/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- R01 NS043946/NS/NINDS NIH HHS/United States
- R01 AG13741/AG/NIA NIH HHS/United States
- P01 AG16570/AG/NIA NIH HHS/United States
- P50 AG05142/AG/NIA NIH HHS/United States
- AG16793/AG/NIA NIH HHS/United States
- P50 AG 16570/AG/NIA NIH HHS/United States
- R01 AG016793/AG/NIA NIH HHS/United States
- R01 AG013741/AG/NIA NIH HHS/United States
- P50 AG016570/AG/NIA NIH HHS/United States
- NS43946/NS/NINDS NIH HHS/United States
- R01 AG010685/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

