Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway
- PMID: 15452145
- PMCID: PMC2172014
- DOI: 10.1083/jcb.200406060
Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway
Abstract
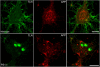
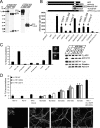
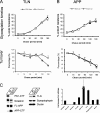
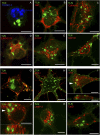
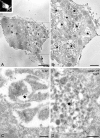
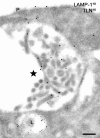
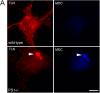
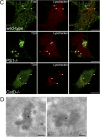
Presenilin 1 (PS1) interacts with telencephalin (TLN) and the amyloid precursor protein via their transmembrane domain (Annaert, W.G., C. Esselens, V. Baert, C. Boeve, G. Snellings, P. Cupers, K. Craessaerts, and B. De Strooper. 2001. Neuron. 32:579-589). Here, we demonstrate that TLN is not a substrate for gamma-secretase cleavage, but displays a prolonged half-life in PS1(-/-) hippocampal neurons. TLN accumulates in intracellular structures bearing characteristics of autophagic vacuoles including the presence of Apg12p and LC3. Importantly, the TLN accumulations are suppressed by adenoviral expression of wild-type, FAD-linked and D257A mutant PS1, indicating that this phenotype is independent from gamma-secretase activity. Cathepsin D deficiency also results in the localization of TLN to autophagic vacuoles. TLN mediates the uptake of microbeads concomitant with actin and PIP2 recruitment, indicating a phagocytic origin of TLN accumulations. Absence of endosomal/lysosomal proteins suggests that the TLN-positive vacuoles fail to fuse with endosomes/lysosomes, preventing their acidification and further degradation. Collectively, PS1 deficiency affects in a gamma-secretase-independent fashion the turnover of TLN through autophagic vacuoles, most likely by an impaired capability to fuse with lysosomes.
Figures


Similar articles
-
Presenilin 1: more than just gamma-secretase.Biochem Soc Trans. 2005 Aug;33(Pt 4):559-62. doi: 10.1042/BST0330559. Biochem Soc Trans. 2005. PMID: 16042544 Review.
-
Degradative organelles containing mislocalized alpha-and beta-synuclein proliferate in presenilin-1 null neurons.J Cell Biol. 2004 May 10;165(3):335-46. doi: 10.1083/jcb.200403061. Epub 2004 May 3. J Cell Biol. 2004. PMID: 15123735 Free PMC article.
-
Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins.Neuron. 2001 Nov 20;32(4):579-89. doi: 10.1016/s0896-6273(01)00512-8. Neuron. 2001. PMID: 11719200
-
Presenilin-1, nicastrin, amyloid precursor protein, and gamma-secretase activity are co-localized in the lysosomal membrane.J Biol Chem. 2003 Jul 18;278(29):26687-94. doi: 10.1074/jbc.m304009200. Epub 2003 May 7. J Biol Chem. 2003. PMID: 12736250
-
Role of presenilin in gamma-secretase cleavage of amyloid precursor protein.Exp Gerontol. 2000 Jul;35(4):453-60. doi: 10.1016/s0531-5565(00)00111-x. Exp Gerontol. 2000. PMID: 10959033 Review.
Cited by
-
Autophagy modulation for Alzheimer's disease therapy.Mol Neurobiol. 2013 Dec;48(3):702-14. doi: 10.1007/s12035-013-8457-z. Epub 2013 Apr 27. Mol Neurobiol. 2013. PMID: 23625314 Review.
-
Super-resolution microscopy reveals majorly mono- and dimeric presenilin1/γ-secretase at the cell surface.Elife. 2020 Jul 7;9:e56679. doi: 10.7554/eLife.56679. Elife. 2020. PMID: 32631487 Free PMC article.
-
Vitronectin induces phosphorylation of ezrin/radixin/moesin actin-binding proteins through binding to its novel neuronal receptor telencephalin.J Biol Chem. 2012 Nov 9;287(46):39041-9. doi: 10.1074/jbc.M112.383851. Epub 2012 Sep 27. J Biol Chem. 2012. PMID: 23019340 Free PMC article.
-
AAV-mediated delivery of an anti-BACE1 VHH alleviates pathology in an Alzheimer's disease model.EMBO Mol Med. 2022 Apr 7;14(4):e09824. doi: 10.15252/emmm.201809824. Epub 2022 Mar 30. EMBO Mol Med. 2022. PMID: 35352880 Free PMC article.
-
Early etiology of Alzheimer's disease: tipping the balance toward autophagy or endosomal dysfunction?Acta Neuropathol. 2015 Mar;129(3):363-81. doi: 10.1007/s00401-014-1379-7. Epub 2015 Jan 3. Acta Neuropathol. 2015. PMID: 25556159 Free PMC article. Review.
References
-
- Annaert, W., and B. De Strooper. 2002. A cell biological perspective on Alzheimer's disease. Annu. Rev. Cell Dev. Biol. 18:25–51. - PubMed
-
- Annaert, W.G., L. Levesque, K. Craessaerts, I. Dierinck, G. Snellings, D. Westaway, P.S. George-Hyslop, B. Cordell, P. Fraser, and B. De Strooper. 1999. Presenilin 1 controls γ-secretase processing of the amyloid precursor protein in pre-Golgi compartments of hippocampal neurons. J. Cell Biol. 147:277–294. - PMC - PubMed
-
- Annaert, W.G., C. Esselens, V. Baert, C. Boeve, G. Snellings, P. Cupers, K. Craessaerts, and B. De Strooper. 2001. Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins. Neuron. 32:579–589. - PubMed
-
- Cai, D., J.Y. Leem, J.P. Greenfield, P. Wang, B.S. Kim, R. Wang, K.O. Lopes, S.H. Kim, H. Zheng, P. Greengard, et al. 2003. Presenilin-1 regulates intracellular trafficking and cell surface delivery of β-amyloid precursor protein. J. Biol. Chem. 278:3446–3454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

