Uterus hyperplasia and increased carcinogen-induced tumorigenesis in mice carrying a _targeted mutation of the Chk2 phosphorylation site in Brca1
- PMID: 15485917
- PMCID: PMC522227
- DOI: 10.1128/MCB.24.21.9498-9507.2004
Uterus hyperplasia and increased carcinogen-induced tumorigenesis in mice carrying a _targeted mutation of the Chk2 phosphorylation site in Brca1
Abstract
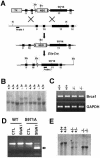
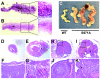
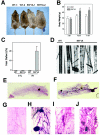
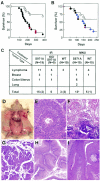
The tumor suppressor BRCA1 contains multiple functional domains that interact with many proteins. After DNA damage, BRCA1 is phosphorylated by CHK2 at serine 988, followed by a change in its intracellular location. To study the functions of CHK2-dependent phosphorylation of BRCA1, we generated a mouse model carrying the mutation S971A (S971 in mouse Brca1 corresponds to S988 in human BRCA1) by gene _targeting. Brca1(S971A/S971A) mice were born at the expected ratio without a developmental defect, unlike previously reported Brca1 mutant mice. However, Brca1(S971A/S971A) mice suffered a moderately increased risk of spontaneous tumor formation, with a majority of females developing uterus hyperplasia and ovarian abnormalities by 2 years of age. After treatment with DNA-damaging agents, Brca1(S971A/S971A) mice exhibited several abnormalities, including increased body weight, abnormal hair growth pattern, lymphoma, mammary tumors, and endometrial tumors. In addition, the onset of tumor formation became accelerated, and 80% of the mutant mice had developed tumors by 1 year of age. We demonstrated that the Brca1(S971A/S971A) cells displayed reduced ability to activate the G(2)/M cell cycle checkpoint upon gamma-irradiation and to stabilize p53 following N-methyl-N'-nitro-N-nitrosoguanidine treatment. These observations suggest that Chk2 phosphorylation of S971 is involved in Brca1 function in modulating the DNA damage response and repressing tumor formation.
Figures
Similar articles
-
Impaired skin and mammary gland development and increased gamma-irradiation-induced tumorigenesis in mice carrying a mutation of S1152-ATM phosphorylation site in Brca1.Cancer Res. 2009 Dec 15;69(24):9291-300. doi: 10.1158/0008-5472.CAN-09-2418. Cancer Res. 2009. PMID: 19996295 Free PMC article.
-
ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency.EMBO J. 2006 May 17;25(10):2167-77. doi: 10.1038/sj.emboj.7601115. Epub 2006 May 4. EMBO J. 2006. PMID: 16675955 Free PMC article.
-
Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair.Mol Cell Biol. 2004 Jan;24(2):708-18. doi: 10.1128/MCB.24.2.708-718.2004. Mol Cell Biol. 2004. PMID: 14701743 Free PMC article.
-
Role of the tumor suppressor gene Brca1 in genetic stability and mammary gland tumor formation.Oncogene. 2000 Feb 21;19(8):1059-64. doi: 10.1038/sj.onc.1203269. Oncogene. 2000. PMID: 10713690 Review.
-
_targeting chk2 kinase: molecular interaction maps and therapeutic rationale.Curr Pharm Des. 2005;11(22):2855-72. doi: 10.2174/1381612054546716. Curr Pharm Des. 2005. PMID: 16101442 Review.
Cited by
-
Brca1 Mouse Models: Functional Insights and Therapeutic Opportunities.J Mol Biol. 2024 Jan 1;436(1):168372. doi: 10.1016/j.jmb.2023.168372. Epub 2023 Nov 17. J Mol Biol. 2024. PMID: 37979908 Free PMC article. Review.
-
The role of BRCA1 in homologous recombination repair in response to replication stress: significance in tumorigenesis and cancer therapy.Cell Biosci. 2013 Feb 6;3(1):11. doi: 10.1186/2045-3701-3-11. Cell Biosci. 2013. PMID: 23388117 Free PMC article.
-
Genetically engineered mouse models for hereditary cancer syndromes.Cancer Sci. 2023 May;114(5):1800-1815. doi: 10.1111/cas.15737. Epub 2023 Feb 14. Cancer Sci. 2023. PMID: 36715493 Free PMC article. Review.
-
Mammary tumorigenesis following transgenic expression of a dominant negative CHK2 mutant.Cancer Res. 2006 Feb 15;66(4):1923-8. doi: 10.1158/0008-5472.CAN-05-1237. Cancer Res. 2006. PMID: 16488990 Free PMC article.
-
BRCA1 deficient mouse models to study pathogenesis and therapy of triple negative breast cancer.Breast Dis. 2010;32(1-2):85-97. doi: 10.3233/BD-2010-0308. Breast Dis. 2010. PMID: 21778574 Free PMC article. Review.
References
-
- Alberg, A. J., and K. J. Helzlsouer. 1997. Epidemiology, prevention, and early detection of breast cancer. Curr. Opin. Oncol. 9:505-511. - PubMed
-
- Barlow, C., M. A. Eckhaus, A. A. Schaffer, and A. Wynshaw-Boris. 1999. Atm haploinsufficiency results in increased sensitivity to sublethal doses of ionizing radiation in mice. Nat. Genet. 21:359-360. - PubMed
-
- Bartek, J., and J. Lukas. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3:421-429. - PubMed
-
- Bell, D. W., J. M. Varley, T. E. Szydlo, D. H. Kang, D. C. Wahrer, K. E. Shannon, M. Lubratovich, S. J. Verselis, K. J. Isselbacher, J. F. Fraumeni, J. M. Birch, F. P. Li, J. E. Garber, and D. A. Haber. 1999. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 286:2528-2531. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
